Table of Contents

Volumetric Analysis Techniques in Solutions and Titrations
Volumetric analysis is an essential technique used to accurately determine the concentration, molar mass, or formula of an unknown compound.
Type of Volumetric Analysis Techniques
There are several general types of volumetric analysis techniques, depending on the nature of the reaction occurring during the titration:
- Acid-Base titrations – involves a reaction between an acid and a base;
- Redox titrations – involves oxidation and reduction between reactants;
- Complexometric titration – involves the reaction resulting in metal-ligand complexation;
- Precipitation titration – involves the formation of a precipitate as a result of a reaction;
Accurate measurement of unknown characteristics
Accurate measurement of unknown characteristics can be obtained through the proper implementation of 3 primary techniques:
- Adequate use of graduated apparatus;
- Precise measurements and weighing of a substance;
- Proper use of indicators.
Important Terms
Before we continue with more specific features of the concept, let’s define several important terms:
- Titrant = standard solution – the solution of a known concentration
- Titrate = Analyte – solution of an unknown concentration
- Indicator – reagent used to determine the endpoint of the titration
Common indicators include the following:
- Phenolphthalein – when we have strong acid and strong base (indicator is colorless in acidic solution and turns pink at basic solutions; as the alkalinity increases, the color changes from pink to dark and bright purple);
- Methyl orange – when we have strong acid and weak base; (indicator is red in acidic solution and yellow in basic solution);
- Phenol Red – colour of the indicator changes from yellow to red and turns into bright pink in alkaline solutions.
- Endpoint = equivalent point – the point at which the indicator changes its colour; comes when the moles of titrant is equal to the moles of an analyte.
- Burette – used to deliver accurate and known volumes of solutions into a volumetric flask
- Volumetric flask – graduated flask used to prepare solutions or used directly during the titration process.
- Pipette – used to deliver a definite volume of a substance into a flask or beaker to prepare a solution of accurate concentration.
Solutions for titration are usually prepared using DI (deionized) water.
Preparation of a standard solution
- Weigh out the required mass of solute
- Dissolve the solute in solvent (usually DI water) in a beaker
- Transfer prepared solution into a volumetric flask
- Mix the solution thoroughly
Titration results are calculated using the following equation:
Ca = CtVt/Va
Where:
Ca – the concentration of analyte
Ct – the concentration of titrant
Vt - the volume of titrant consumed during the titration
Va – the volume of analyte delivered using a pipette
Let’s consider the following problem:
Sample Problem:

To calculate the molar mass of an unknown sample, we should determine the number of moles of the unknown sample. Then, we just divide the mass of our analyte in grams by the amount of analyte in moles, and we get the molar mass.
M = m/n
Solutions
There are various types of solutions, the simplest one of which is a solution that consists of 2 substances: a solute and a solvent.
Solute – a substance that is present in a smaller amount.
Solvent – a substance that is present in a larger amount and in which the solute is dissolved.
Any interactions between the solute and solvent molecules are defined by the term solvation.
If the solvent is water, solvation process is now called hydration, and the solution is referred to as an aqueous solution.
In terms of solubility, there is a limit to the amount of solute that can be completely dissolved in a solvent. Although gas mixtures are an exception since solute and solvent molecules can be mixed in any proportion. This exception can also be valid for particular liquid mixtures (e.g., ethanol and water).
2 major terms describe the solution in terms of the amount of dissolved solute:
- Saturated solution – when the maximum amount of solute is dissolved in a solution;
- Unsaturated solution – when the limit to the amount that can be dissolved in a solution is not reached; therefore, more solute can be dissolved in the solution.
Read more about Empirical and Molecular Formula
Concentration
Concentration is a term that describes the amount of solute dissolved in a specific amount of a solution.
We can use the following equation to calculate the concentration of a solution:
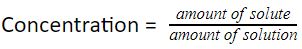
The amount of solution is the sum of the amount of solute and the amount of solvent.
Concentration can be expressed in various units, including the following: g/L; mol/L
To calculate the percent concentration of a solution, we simply multiply the calculated concentration by 100%.
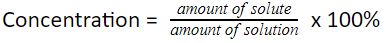
We can calculate weight/weight (w/w), volume/volume (v/v), or weight/volume (w/v) percent compositions of the solutions.
In the case of w/w percent composition, we just divide the mass of solute by the mass of solution and multiply it by 100%.
In the case of v/v percent composition, we just divide the volume of solute by the volume of solution and multiply it by 100%.
In the case of w/v percent composition, we just divide the mass of solute by the volume of solution and multiply it by 100%.
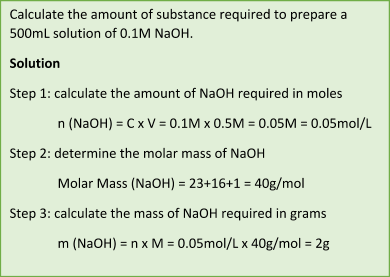
Molarity
Molarity – a term used to define the concentration in moles of solute per litre of solution. Concentrations that are characterized using molarity are called molar concentrations and are abbreviated as M.
For instance, if the concentration of a solution is denoted as 2.75M:
- It is a 2.75 molar solution;
- Molarity of this solution is 2.75 moles of solute per litre of solution (or simply 2.75mol/L);
Molarity can be calculated using the following equation:
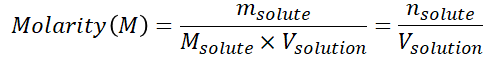
Molality (m) – a term used to define the number of moles of solute dissolved in one kilogram of solvent.
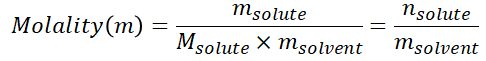
NOTE: mass of solvent is in kilograms, not in grams
Frequently Asked Questions
What is redox titration?
Redox titration is a type of volumetric analysis in which a given solution is titrated against another such that a redox reaction takes place between the two. This is done mostly for organic solutions. Indicators used are diphenyl benzidine, diphenylamine and sodium diphenylamine sulphonate, etc.
What are the different indicators used in acid-base titrations?
Indicators used in acid-base titrations are phenolphthalein, methyl red, bromophenol blue, bromocresol green and purple and methyl orange etc.
What is a burette and its use?
A burette is a graduated glass tube with a stopper at one end and is open at the other end. It is made up of glass and used in volumetric analysis such as acid-base titrations, redox titrations etc.
What are the SI units for molarity and molality?
As molarity is no. of moles dissolved in one litre of solution and molality is no. of moles dissolved per 1000 gram of solvent, the SI units for molarity and molality are mol per litre and mol per kg, respectively.





