Table of Contents
Empirical Formula
To determine the formulas for different compounds, scientists did not use the periodic table, rather formulas were determined through the quantitative analysis which determines the percent composition of a compound. After identification of the relative mass of each atom in a particular compound, one can determine the empirical formula of that compound.
Therefore, the empirical formula can be defined as the simplest whole-number ratio of the constituent atoms of a sample compound. Let's learn more about the empirical and molecular formula!
Let’s consider several examples to better understand the idea of the empirical formula.
Sample Problem:

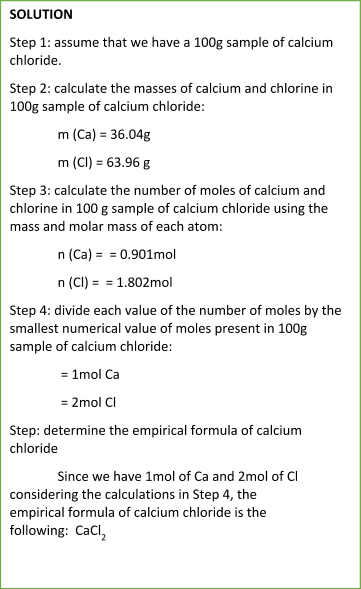

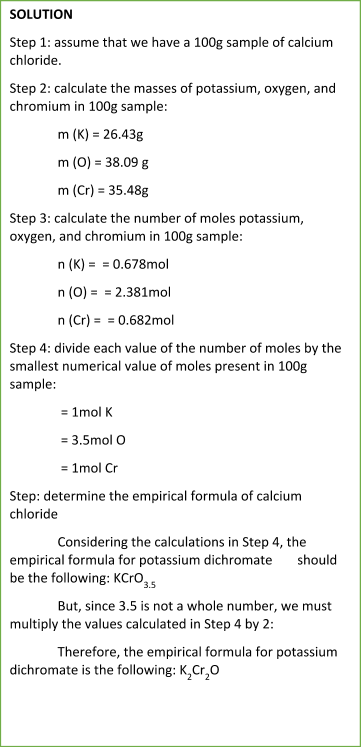
NOTE
You must use always the whole numbers for determining the empirical formula of a compound.
If you are asked to write the empirical formula for the following compounds:
C2H4, C6H14, C6H12O6
You just find the formula with the simplest whole-number ratio.
For example:
Empirical formula for C2H4 would be CH2
Empirical formula for C6H14 would be C3H7
Empirical formula for C6H12O6 would be CH2O
Even though the empirical formula of ionic or molecular compounds shows the simplest whole-number ratio of its elements, it cannot depict the actual number of each type of atom in a molecular compound.
Read more about Formulae Equations and Amount of Substance
Molecular Formulas
For that reason, we need molecular formulas to get more detailed information about molecular composition.
Let’s consider the following problem to get the idea of a molecular formula.
Sample Problem:
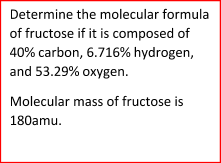
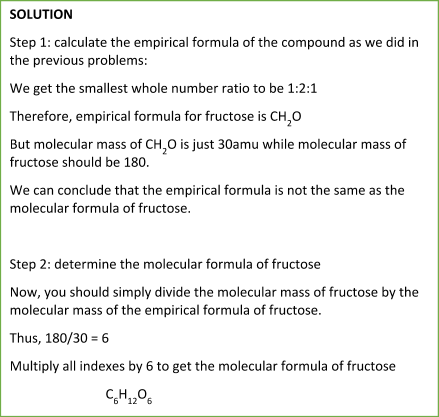
Summary
The empirical formula of a compound can be defined as the simplest whole-number ratio of the constituent atoms of that specific compound.
The empirical formula only depicts the information about the constituent atoms of the molecule but not the whole information about the compositions.
It is quite easy to determine the empirical formula of a compound. General steps for determination are provided below:
If you are given the percent composition of a specific compound but there is no information about the mass of the sample, the first thing that you do is that you assume the mass of that specific compound to be 100g.
Step 1: Assume that the mass of a compound is 100g, so it is easier to calculate the mass of each component in the molecule.
Step 2: since you have assumed that the mass of the compound is 100g, you just rewrite the values that were given in percentages but the units are now grams (do not get confused, you just calculate the mass of the atoms by multiplying the mass of the sample by the given percentage and then dividing by 100; since the mass is assumed to be 100g, there is no point in multiplying by 100 and then dividing by 100; that is why you leave the percentage values as they are; you just change the units).
Step 3: Calculate the number of moles for every atom present in the molecule. To do so, you simply divide the mass of an atom calculated in Step 2 by the molar mass of that particular atom.
Step 4: Determine the smallest numerical value of moles from the ones that you have calculated in Step 3.
Step 5: Divide each value calculated in Step 3 by the smallest value determined in Step 4. By this, you get the ratio of the atoms that are present in your molecule
Step 6: Write the empirical formula considering the values that you have calculated in Step 5.
NOTE:
In case if the problem provides information about the mass of the sample compound, you are no longer allowed to assume that the mass of the sample is 100g; rather, you take the given value and continue the calculation steps.
To calculate the mass of each atom present in the molecule when you are given the specific mass of a sample, you simply multiply the given mass of a sample by the percentage of a particular atom and divide by 100.
For example, there is a 23g sample that consists of 12% potassium. To calculate the mass of potassium in the sample you multiply 23 by 12 and divide by 100. You will get 2.76g.
As long as you calculate the mass of each atom present in a given sample, you can follow the same steps (from Step 3 above) to determine the empirical formula.
On the other hand, the molecular formula can be used to get detailed information about the atomic composition of any compound.
When you are asked to determine the molecular formula of a compound, you are most probably given the molecular weight of that particular compound along with the percent composition.
To do so, you should follow the following steps:
Step 1: Determine the empirical formula of a compound.
Step 2: Calculate the molecular weight of the determining empirical formula.
Step 3: Divide the given value for the molecular weight of the sample compound by the calculated molecular weight of the empirical formula.
Step 4: multiply the indexes of the empirical formula by the number that you have calculated in
IMPORTANT NOTE:
Be aware that neither the empirical formula nor the molecular formula should contain decimal indexes. Rather, all the indexes must be whole numbers. So, in case if you get the ratio of the elements consisting of decimal numbers, you just multiply the values so that you get the whole numbers.
Frequently Asked Questions
What is the empirical formula of a compound?
The empirical formula of a compound tells us about the simplest ratio between constituent elements of a compound expressed in whole numbers. There may be the same empirical formula for more than one compound. E.g., the empirical formula for ethene is CH2.
Can a molecule have the same empirical and molecular formulae?
Yes, a molecule may have the same empirical and molecular formulae. For example; CO2 has the same molecular and empirical formula because one molecule of carbon dioxide contains carbon and oxygen in the simplest whole number ratio i.e., 1:2
What are the limitations of the empirical formula?
The empirical formula can not give us the exact identity of a compound because more than one compound can have the same empirical formula. It also does not show the exact number of atoms of a particular element present in a compound.
Can an element in a chemical formula get a decimal index?
No, an element can get a decimal index neither in the empirical formula nor in the molecular formula. It must be shown in a whole number.
References:
OpenStax. (2015). “OpenStax, Chemistry.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





