Table of Contents
Introduction to Polymerization
A polymer is a substance made up of large molecules which have repeating subunits. Polymers are both natural and artificial, both types having unique physical properties like toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semi-crystalline structures rather than crystals. Historically, the use of natural polymers like wool (keratin), cotton and linen fibers (cellulose) for garments and paper reed (cellulose) for paper has been widespread over the past few thousand years. The most easily recognizable form of polymer in society, i.e. rubber has been in use for quite a long time. The latex sap of “caoutchouc” trees (natural rubber) was known to Olmec, Maya, and Aztec civilizations who used the natural rubber to make balls, waterproof textiles, and containers.
The advent of synthetic chemistry in the polymerization process can be traced to the vulcanization of natural rubber. This eventually opened the floodgates for research on polymers and at present, polymers have become an integral part of the society as products ranging from toys to household items and from sports to spaceships make use of polymers.
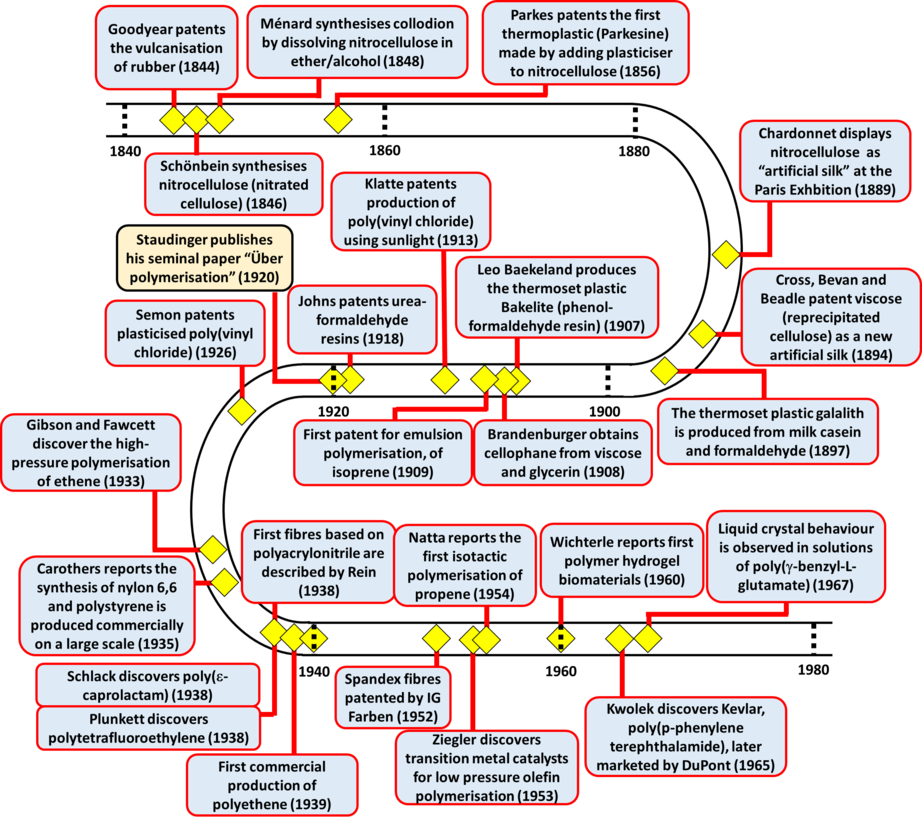
Image source: Wikipedia commons
Classification of Polymerization According to Reaction Kinetics and Mechanism
Polymerization reactions can be classified into two broad classes according to the kinetics the reactants follow - step-growth polymerization and chain polymerization. The former proceeds via a direct combination of chains of monomers while the latter proceeds via the addition of monomers to the chain one at a time.
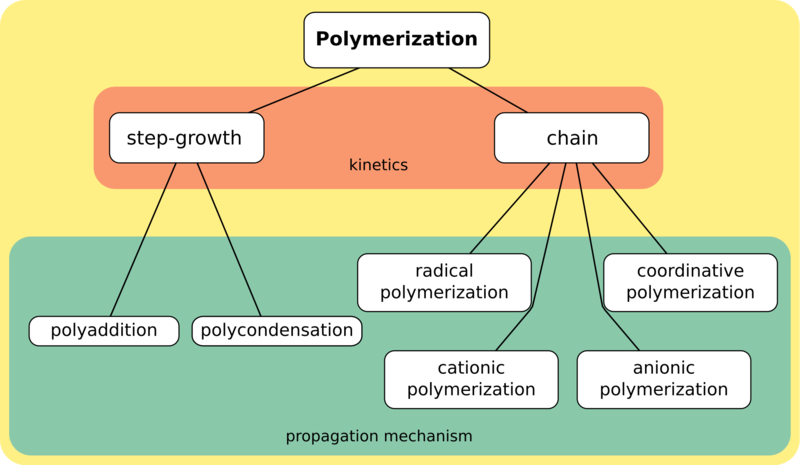
Image source: Wikipedia commons
Classification of Polymerization According to Reaction Types
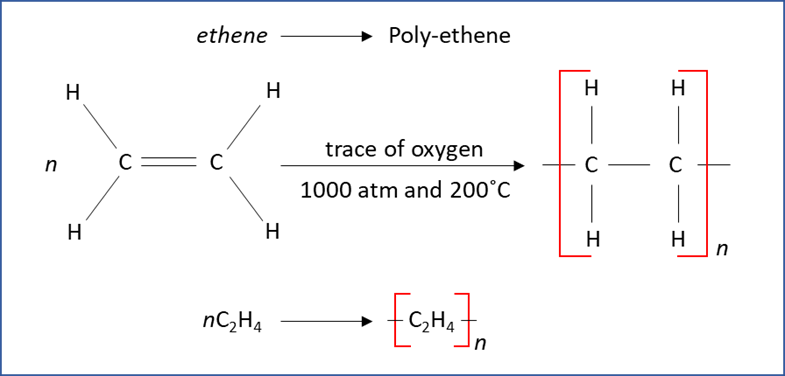
Polymers are formed by two types of reactions: addition polymerization and condensation polymerization. If the monomer has a C=C bond, it undergoes addition polymerization. For example, a large number of ethene molecules react with each other under specific conditions to form the polymer polyethylene. In this reaction, the π-bonds are broken, and the monomers link together. For a condensation reaction to take place, two different functional groups react with each other. The formation of polyester terylene by the reaction of benzene-1,4-dicarboxylic acid and ethane-1,2-diol is an example of condensation polymerization.
Addition polymerization - Unsaturated molecules react with each other under specific conditions to form polymer molecules. The small units that react together to make the polymer are called monomers. For example, up to 10,000 molecules of ethene react to form the polymer polyethylene.
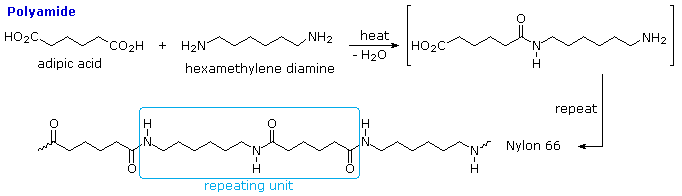
Condensation polymers - These polymerizations often occur with the loss of a small byproduct, such as water, and typically combine two different components in an alternating structure.
Photopolymers - Polymers that change their properties according to the light incident on them. Most often, cross-linking occurs with exposure to light. Curing is a process to convert a mixture of monomers, oligomers, and photoinitiators into a hardened polymeric material. There exist two possible modes of photoinitiation - direct light absorption by the reactant monomer (direct photopolymerization), or light absorption by a photosensitizer which further transfers energy to the monomer.
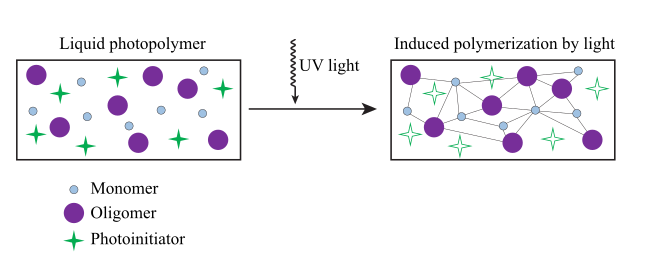
Image source: Wikipedia commons
Microstructure of Polymers
The elasticity, strength, and durability of polymers largely depend on the microstructure which can be defined as the physical arrangement of monomer residues along the backbone of the chain. Thermoplastics are usually branched and unbranched polymers, many elastomers have a wide-meshed cross-linking between the "main chains". Thermosets have close-meshed crosslinking.
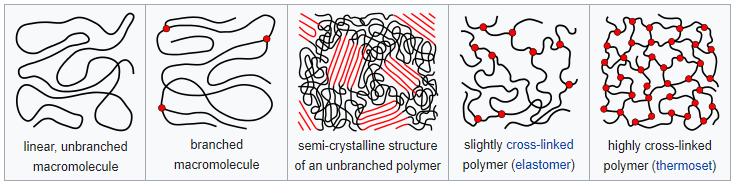
Image source: Wikipedia commons
Modern Techniques for Polymerization
With the advancement of technology, new methods of polymerization have emerged. The rapidly changing fields of polymerization are, however, marked by some landmark techniques that have become an integral part of modern polymer studies both in academia and industry.
Ring-opening polymerization - A lot of modern-day commercially relevant polymers are produced through the ring-opening technique in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer.
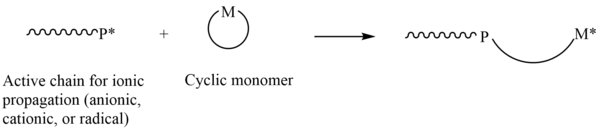
Image Source: Wikipedia commons
Sequence-Controlled Polymerization - Controlling the sequence in which the monomers are added during polymerization leads to a sequence-controlled polymer. These are essential macromolecules and find applications in biological polymerization, solid-phase synthesis, and radical/non-radical polymerizations. Such polymerizations involve the use of protecting groups and deprotection reagents so that the direction and sequence of monomers to be added, can be controlled.
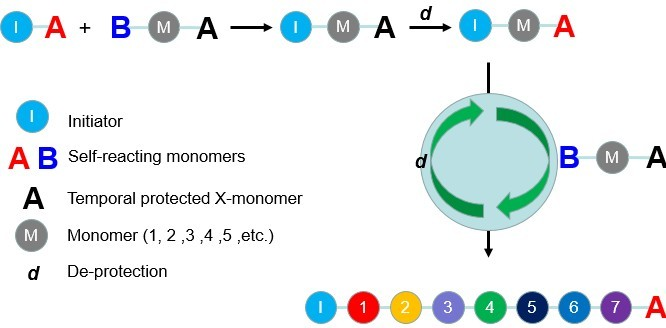
Image Source: Wikipedia commons
Plasma Polymerization - Gaseous or liquid monomer, often containing a vinyl group, is activated or fragmented to initiate polymerization. The monomers should be small ionizable species in this technique. The formation of plasma polymers follows a cyclic step-growth mechanism and the physical properties of plasma polymers depend greatly on the reactor design, chemical, and physical characteristics of the substrate on which the plasma polymer is deposited. Most of the plasma polymers also contain free radicals and have internal stress which leads to their cracking if the deposited layer is too thick. These polymers have great adhesion quality and are insoluble and infusible.
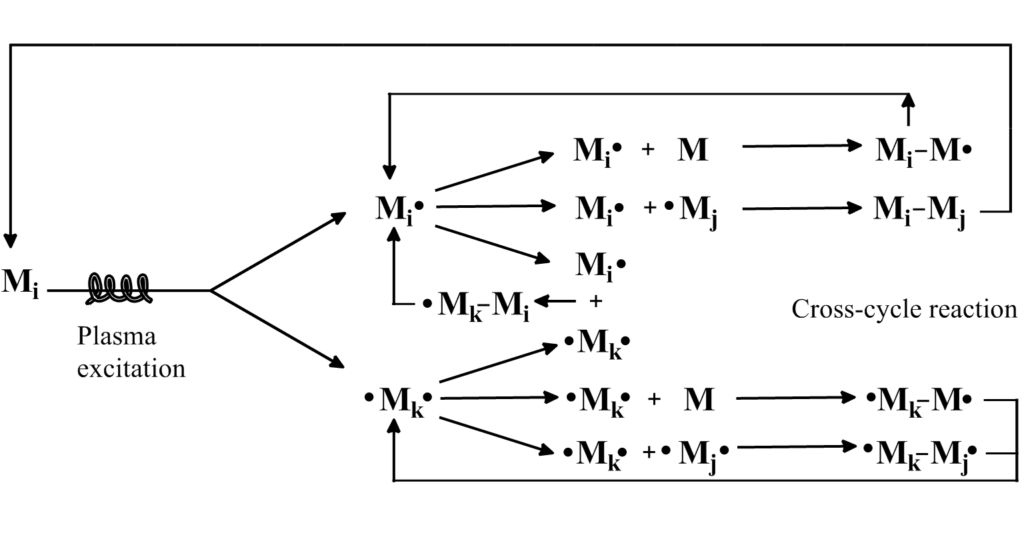
Image Source: Wikipedia commons
Applications of Polymers
Polymerization reactions are used to manufacture a wide variety of materials that cater to almost every walk of life. Some of the prominent uses are:
- Clothing, tents, shelters, umbrella, sports shoes, balls, etc.
- Electronic gadgets, OLEDs, CDs, OFETS, etc.
- Packaging materials
- Construction materials like pipes, sealing, flooring, etc.
- Paints, glues, lubricants
- Medical instruments and accessories
- Money: polymer banknotes and payment cards.
Biodegradability and Disposal of Polymers
In addition, polymers are highly resistant to chemical attacks. Used polymers are disposed to landfill sites which takes up a lot of valuable space. These polymers also pollute the environment as it does not degrade. Polyalkenes are chemically inert and decompose very slowly in the environment. Polychloroethene is less inert when compared to polyethylene because the C-Cl bond can be attacked by alkalis and undergo rupture in the presence of sunlight.
Some safe ways of polymer disposal are:
- Combustion for energy production.
- Recycling.
- Feedstock for cracking.
- Bacterial fragmentation.
Frequently Asked Questions
What is polymerization?
Polymerization is a process in which small subunits known as monomers are joined together to form large molecules. A polymer contains repeating subunits (monomers) that form a long chain. These monomers act as building blocks of macromolecules i.e., DNA, proteins, polysaccharides etc.
What are the steps of the polymerization process?
Polymerization takes place in three steps: initiation, chain elongation also known as propagation and termination. Each step may involve different enzymes or catalysts to take place.
What is the mechanism of polymerization?
Polymerization can take place in either of two ways: condensation reaction or addition reactions. In condensation reactions, monomers join each other and a small molecule is eliminated as a by-product, while in addition reactions, no by-products are formed.
What is condensation polymerization?
In condensation polymerization, when two monomeric subunits join together, a small molecule (i.e., water) is eliminated as a by-product. Most biological polymers are formed by this reaction. For example; when two amino acid residues join together, a peptide bond is formed along with the elimination of one water molecule.
References:
- Journal of Macromolecular Science: Part A - Chemistry. 15 (7): 1279–1287.
- https://ncert.nic.in/textbook/pdf/lech206.pdf
- https://www.britannica.com/science/addition-polymerization
- https://www.britannica.com/science/condensation-polymerization
- Sperling, L. H. (Leslie Howard) (2006). Introduction to physical polymer science. Hoboken, N.J.: Wiley. p. 10. ISBN 978-0-471-70606-9.





