Table of Contents
Key Information & Summary

- Polymers are macromolecules made up of repeating units called monomers.
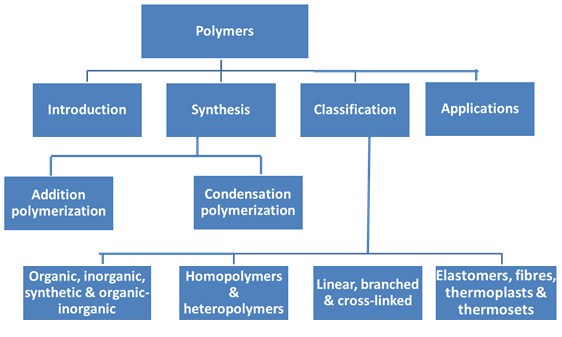
- Polymers can be synthesized through two types of polymerization reactions i.e. addition and condensation polymerization.
- Polymers exist as amorphous or crystalline solids.
- Polymers can be classified as organic, inorganic, synthetic and mixture of organic-inorganic polymers.
- Polymers may be classified as heteropolymer or homopolymers.
- Polymers may be categorized as linear, branched and cross-linked based on their structures.
- Polymers are classified as Elastomers, fibres, thermosplasts and thermosets based on their properties.
- Organic polymers including proteins, carbohydrates and DNA perform significant functions in living beings.
- Inorganic polymers like polysiloxanes are used in making electrical insulations and protective coatings.
- Synthetic polymers are used to make plastic bags, bottles, cables, pipes etc.
Introduction
Polymers are the long chain macromolecules that are made up of many repeating units called monomers. The concept of polymers was first introduced by a German chemist, Hermann Staudinger, who elaborated the polymeric structure of rubber formed by repeating isoprene monomer. Stuadinger proposed that polymers are made up of 10,000 or more atoms.
Synthesis of polymers
The reaction involved in synthesis of polymers is called polymerization that links various monomers together. There are two types of polymerization i.e. addition (chain growth) polymerization and condensation (step growth) polymerization.
Addition polymerization: During addition polymerization unsaturated monomers are added to form long chain of polymer but there is no release of any atom or molecule. The products of condensation polymerization are called thermoplastics.
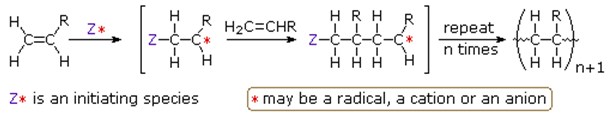
Addition polymerization requires radical initiators or Lewis acid/base as catalysts. Molecular weight of addition polymers is integral multiple of monomer’s molecular weight. Examples of addition polymers include polyethylene, polystyrene, PVC etc. There are 4 types of addition polymerization reaction:
- Radical polymerization in which the initiating species (Z*) is a radical while the site of reactivity (*) is a carbon radical.
- Anionic polymerization in which the initiating species (Z*) is a nucleophile while the site of reactivity (*) is a carbanion.
- Cationic polymerization in which the initiating species (Z*) is an acid while the site of reactivity (*) is a carbocation.
- Coordination catalytic polymerization in which the initiating species (Z*) is a transition metal complex while the site of reactivity (*) is a terminal catalytic complex.
Condensation polymerization: During condensation polymerization, bifunctional monomers are linked together with the release of a small molecule like water, HCl, ammonia etc. as byproducts.
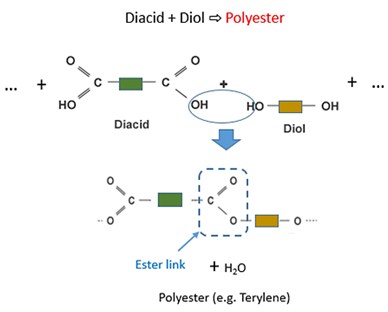
Condensation polymerization reactions are catalysed by mineral acids and bases. The products of condensation polymerization are called thermosets. Examples of condensation polymers include synthetic polymers like polyester, nylon etc. and organics polymers like proteins and carbohydrates. Condensation polymers form slowly and are usually low molecular weight polymers.
Read more about Polymers and Polyamides
Physical properties of polymers
Polymers exist as amorphous and crystalline solids. Crystalline solids have high melting points, rigidity, tensile strength and opacity. On the other hand amorphous solids have lower melting points and get easily deformed. . The semi-crystalline solids have crystalline lamellae and amorphous regions that are linked via tie molecules.
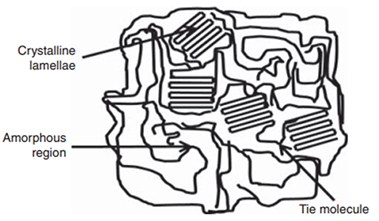
The number of repeating units in a polymer refers to degree of polymerization of that polymer. Increased degree of polymerization, branching and interchain branching promotes crystallinity in polymers thereby increasing their tensile strength. For example, there is a lot of interchain bonding between the fibres of cellulose that gives it a stable rod like structure.
Classification of Polymers
Organic, inorganic, synthetic and mixture of organic-inorganic: Polymers can be organic, inorganic and synthetic in nature. Organic polymers include proteins (made up of amino acid monomers), DNA (made up of nucleotide monomers), starch or cellulose (made up of glucose monomers) etc. Inorganic polymers are made up of inter-linkages between multivalent atoms except carbon. They are also present in nature like talc, mica, clay etc. Inorganic polymers are stable at high temperatures and show elastomeric properties at low temperature. Important inorganic polymers include borates that are salts of oxyacids of boron e.g. borax that is used in manufacturing glass. Synthetic polymers include plastics, glass, concrete etc. Some polymers are mixture of organic-inorganic compounds, e.g. silicone. It is a polymer having silicon and oxygen atoms as a backbone. Various organic groups attach to this inorganic backbone. Low molecular weight silicones are oily or greasy while high molecular weight silicones are elastic and rubbery.
Homopolymers and heteropolymers: On the basis of type of monomers, polymers can be classified as homopolymers and heteropolymers. Homopolymers have only one type of repeating monomer while the heteropolymer is made up of two or more types of monomers.
Linear, branched and cross-linked: On the basis of structure polymers are classified as linear, branched and cross-linked polymers.
Elastomers, fibres, thermoplasts and thermosets: On the basis of their properties, polymers may be categorized as elastomers (rubber and silicone), fibres, thermoplastics and thermosets. Thermoplastic polymers (e.g. PVC and polystyrene) can be repeatedly softened by heating and remoulded into new shapes. Thermosets (e.g. bakelite) undergo a permanent change on heating such that their shape cannot be changed anymore. Heating creates strong cross links that cannot be reversed.
Applications of polymers
Natural polymers find various applications in living things. For example, cellulose and lignin are important parts of plants. Proteins involved in structure and function, starches having a role in providing energy and DNA containing genetic information is also the example of natural polymers found in almost every living thing. Natural rubber that is a polymer of isoprene,
Inorganic polymers are also very useful polymers. Polysiloxanes are the most useful inorganic polymers because of their high permeability to gases, flexibility and low surface energy. They are used as water repellents, electrical insulators, protective coatings, adhesives and anti-foaming agents. Often polysiloxanes are mixed with other polymers to improve hydrophobicity and resistance.
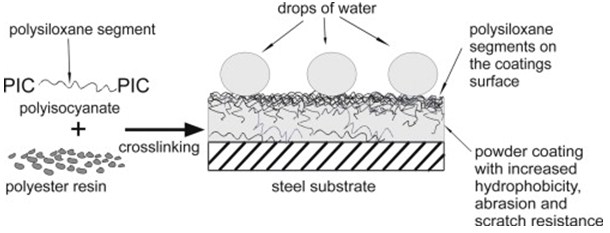
Synthetic polymers are used as packaging materials, coatings, hydrogels and teeth fillers etc. Polyethylene, a polymer of ethylene, is widely used for making plastic bags and bottles. polypropene is used for making crates and ropes while polychloroethene and polyvinylchloride are used for making water pipes. Polypropylene, a crystalline and thermoplastic polymer, is used in textile industry for making moulded objects. Vulcanized rubber is a synthetic form of rubber in which natural rubber is treated with a vulcanizing agent (sulphur) to increase cross-lining in the natural polymer. Vulcanized rubber has more strength and resilience than natural rubber and is used to make shoe soles, toys, hockey balls, rubber hoses etc.

Graphical Summary
Frequently Asked Questions
What are polymers?
Polymers are natural or synthetic macromolecules made up of repeating smaller units called monomers. They may exist as crystalline or amorphous solids.
How are polymers synthesized?
They are synthesized by addition or condensation reactions. In addition reactions, multiple unsaturated molecules react to form a long chain without releasing any smaller molecules. In contrast, in a condensation reaction, two molecules react to form a bond and release a smaller molecule in the process.
How are polymers classified?
Polymers are classified as organic (proteins, nucleic acids etc.) and inorganic (Borax), homopolymers (having one type of monomer) and heteropolymers (having two or more types of monomers).
What are the uses of polymers?
Polymers are used as water repellents, electrical insulators, adhesives, anti-foaming agents, protective coatings, packaging materials, hydrogels, teeth fillers and for making plastic bags, bottles and water pipes.
References for further reading
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/polymers.htm
https://www.britannica.com/science/polymer
https://www.britannica.com/topic/inorganic-polymers-1462212
https://science.howstuffworks.com/plastic3.htm
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





