Table of Contents
Introduction
Nitrogen and Sulphur are two elements that constitute a major role in nature and human activities. Nitrogen in its gaseous form is present in the atmosphere at a concentration of 77% and is relatively unreactive in this form. Despite that, the Nitrogen cycle plays a pivotal role in maintaining the balance of life on earth. Sulphur is the 10th most abundant element in the whole universe. Naturally, sulphur occurs as a yellow crystalline substance with a chemical formula S8, wherein these Sulphur atoms bond to each other in a cyclic form. The cyclo-octasulpur is the most abundant allotrope though sulphur rings with 6, 7, 9-15, 18 and 20 atoms are also known. In its pure form, sulphur has no smell, but nearly all sulphur compounds have a characteristic stinky smell.
Nitrogen Compounds
Nitrogen gas is inert because it contains a triple bond between the two nitrogen atoms. Nitrogen atoms combine to form molecules with three pairs of shared electrons called triple covalent bonds. Nitrogen has 5 electrons in its outermost shell and needs three more to reach the stable configuration. Hence, two nitrogen atoms combine to form a nitrogen molecule where three pairs of electrons are shared.
The triple covalent bonding is very strong, and the bond energy is almost 1000 kJ/mol. Under ordinary conditions, it does not react with oxygen in the atmosphere, but these can be reacted under laboratory conditions to produce nitric and nitrous oxides. Nitrous oxide can get further converted to nitric acid.
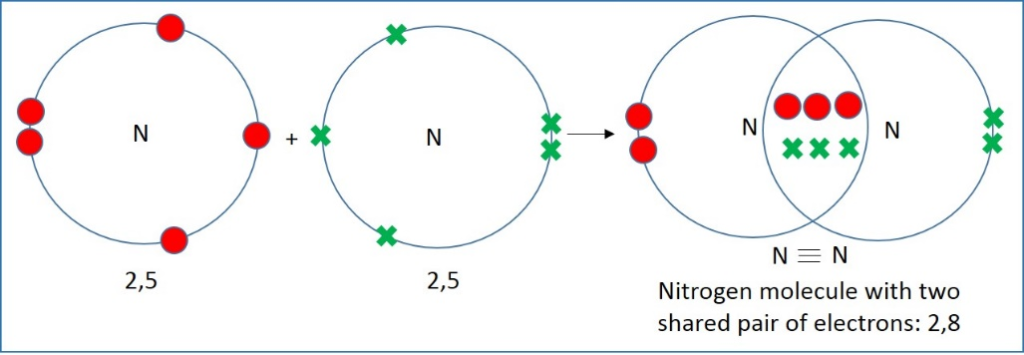
Bonding in Nitrogen Gas
Nitrogen and oxygen react in the presence of thunderstorms. Lightning acts as the source of activation energy for this reaction. This reaction produces nitric oxide, which further reacts with oxygen and water to naturally produce nitric acid, which is absorbed by the soil. This is further used by plants to fix nitrogen.
Ammonia Production
Ammonia is synthesised using Haber’s process and the reaction is,
N2 (g) + 3H2 (g) ⇋ 2NH3 (g) ΔHr=-92 kJ/mol
This reaction takes place in the presence of iron.
When ammonia is dissolved in water, it forms ammonium hydroxide, wherein a positively charged ammonium ion exists with a negatively charged hydroxide ion.
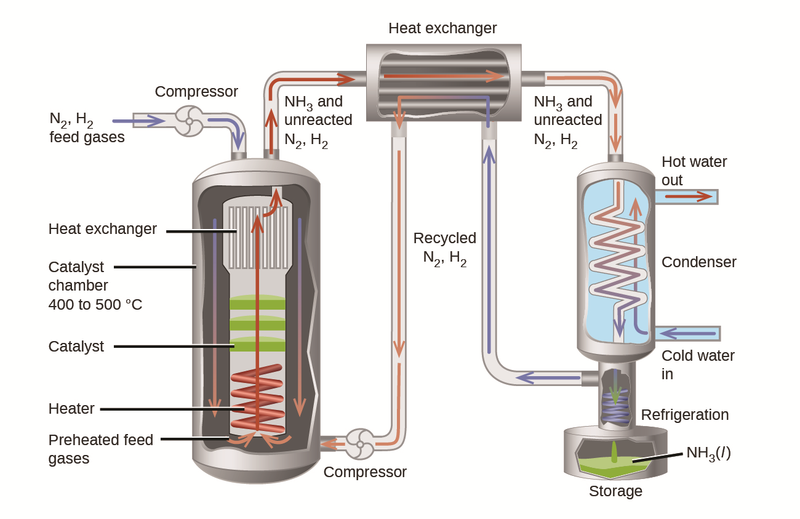
Uses of Ammonia and its Derivatives
Ammonium salts are widely used in fertilisers to replace nitrogen in the soil after each harvest. Some ammonium salts used in fertilisers are NH4Cl (ammonium chloride), NH4NO3 (ammonium nitrate), (NH4)3PO4 ammonium phosphate and ammonium sulphate (NH4)2SO4. These ammonium salts replenish nitrogen deficiency in the soil after every harvest.
Ammonium nitrate is manufactured from ammonia and nitric acid in a simple one-step reaction.
NH3 (aq) + HNO3 (aq) → NH4NO3 (aq)
The ammonium nitrate solution is evaporated, and the resultant solid is melted. This molten solid is sprayed into a tower with air blown into it to make solid pellets.
Concentrated nitric acid is used to make explosives such as trinitrotoluene (TNT). It is also used in manufacturing detergents, dyes, nylon, etc.
Detection of Ammonia
Since ammonia is an important compound for commercial purposes, its detection is of prime importance. Many commercially important compounds can be converted to ammonia in simple steps, thereby facilitating their detection.
For example:
Ammonium ions react with a warm sodium hydroxide solution to form ammonia gas.
NH4+(aq) + NaOH(aq) → NH3(g) + Na+ + H2O
Ammonium chloride and calcium hydroxide react in their solid states when heated and produce ammonia gas.
2NH4Cl (s) + Ca(OH)2 (s) → CaCl2 (s) + 2H2O (l) + 2NH3 (g)
Ammonia is a colourless gas with a pungent smell. This gas turns red litmus paper blue.
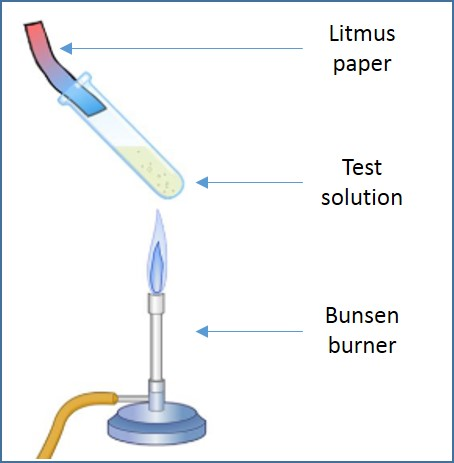
Environmental problems caused by Nitrogen Compounds
1. Nitrogen oxides are air pollutants and cause smog.
2. Nitrogen oxide catalyses the oxidation of sulphur dioxide, which in turn reacts with rainwater to produce acid rain.
3. Nitrate fertilizers promote algae growth in water bodies, leading to eutrophication, leading to the death of aquatic life and the imbalance in the ecosystem.
4. Nitrates in drinking water are believed to cause blue baby syndrome and stomach cancer.
Sulphur Compounds
When burnt in the air, sulphur melts into a blood-red coloured liquid, producing a blue flame. The gas formed in this process is sulphur dioxide. This pungent and irritating gas is soluble in carbon disulphide but not in water.

Solid sulphur (yellow) burning in air
In a +2 oxidation state, sulphur forms nitrides, fluorides, chlorides, bromides, and oxides of different compositions. In its -2 oxidation state, sulphur forms sulphides with elements having electronegativity lower than itself.
Polycations of sulphur are produced by the reaction of sulphur with mild oxidising agents in a strongly acidic solution.
The reactions of hydrogen and sulphur produce hydrogen sulphide, and when it is dissolved in water, the solution becomes mildly acidic. Both H2S and HS- are highly toxic to mammals since they bind to haemoglobin and reduce its oxygen-carrying capacity.
Catenation is a distinctive property of sulphur by virtue of which it forms chains by binding with itself. Such polysulphides are produced by reducing elemental sulphur wherein the terminal sulphur is negatively charged.
Sulphur also participates in organic reactions to produce a plethora of organosulphur compounds. Some notable compounds in this category are allicin (found in garlic), cysteine (amino acid), dibenzothiophene (a component of crude oil), and penicillin (an antibiotic).
Apart from these, sulphur also forms many metal sulphides, pnictides, halides, oxyhalides, oxides, oxoacids and oxoanions are important classes of sulphur compounds.
In sulphur, single bonds are favored, and catenation is easier, which leads to several allotropes.
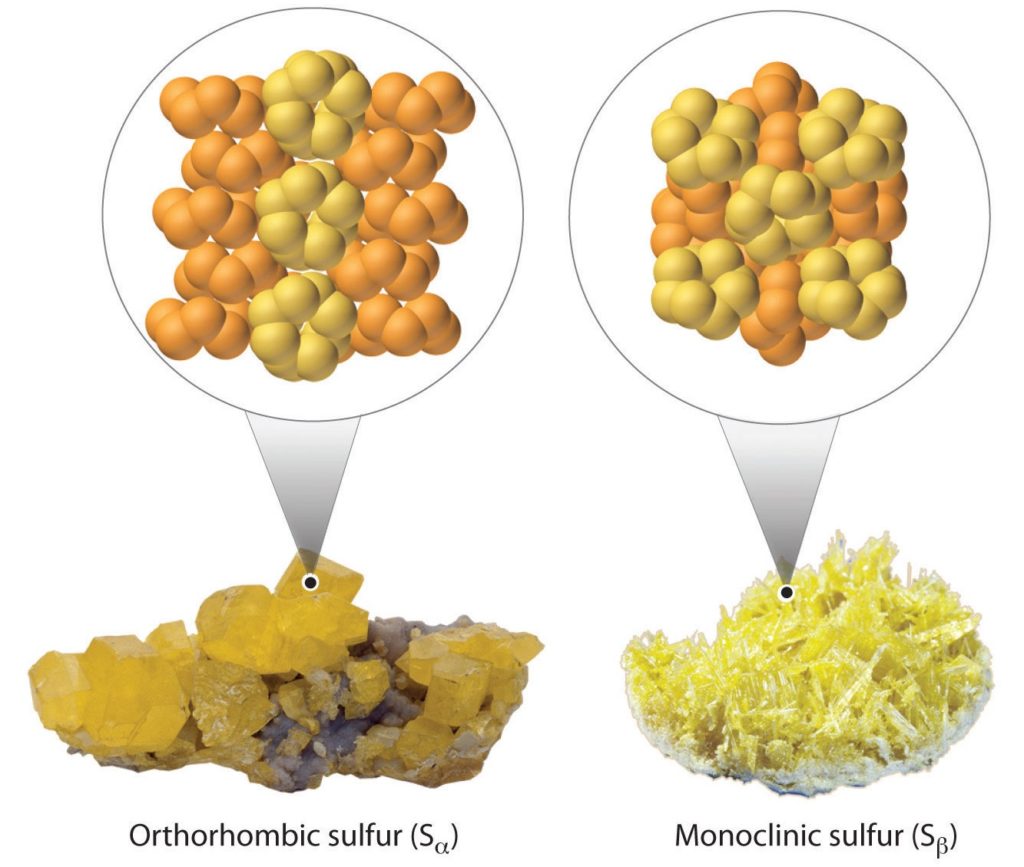
Orthorhombic and monoclinic allotropes of sulphur
Uses of Sulphur Compounds
1. Sulphuric acid is the most widely produced sulphur compound accounting for up to 85% of the sulphur used in industries.
2. Sulphur is used to cross-link organic polymers in natural rubber by the process of vulcanization.
3. Sulphites are used to preserve dry fruits and produce bleach papers.
4. Sulphates are used as surfactants and detergents.
5. Gypsum (calcium sulphate) is used in Portland cement and fertilizer.
6. Elemental sulphur is used in fungicides and pesticides.
7. Sulphur in various forms is used in pharmaceuticals, especially for treating skin diseases and other bacterial infections
Environmental Problems caused by Sulphur Compounds
1. Harmful sulphur dioxide is formed when fossil fuels such as coal, petrol and natural gas are burned. Volcanic activity also releases sulphur dioxide.
2. Acid rain is caused due to sulphur trioxide. Statues, buildings and metallic structures are also affected by acid rain. Statues made of carbonate rock are corroded, and steel is also corroded due to the presence of iron.
3. Hydrogen sulphide causes inhibition of haemoglobin function.
4. Sulphur compounds increase soil pH, thereby leading to an imbalance in microbial growth.
Read more about Compounds, Formulae, and Equations
Frequently Asked Questions
What are the different industrial nitrogen compounds?
Different industrial nitrogen compounds include nitrogen gases (nitrous oxide, nitric oxide), nitrogen fertilizers, nitric acid (HNO3), Ammonia gas and different other nitrates and nitrites (e.ge., sodium nitrate sometimes used for meat preservations).
What are different ammonia fertilizers?
Different ammonia fertilizers are; ammonium chloride (NH4CL), ammonium phosphate ((NH4)3PO4), ammonium nitrate (NO3NH4) ammonium sulphate ((NH4)2SO4) and ammonia (NH3) etc.
How ammonia is produced commercially?
On a commercial scale, ammonia is prepared by the well-known Haber’s process. In this process, atmospheric nitrogen is combined with methane (CH4/ natural gas) in the presence of a metal catalyst (i.e., iron) at a temperature of 450 ℃ and 200 atm (atmospheric pressure).
What are the uses of Sulphur compounds?
The most important Sulphur compound is sulphuric acid which is also known as the king of chemicals. Other Sulphur compounds include sulphates (e.g., calcium sulphate or gypsum used in cement manufacturing, ammonium sulphate (an important nitrogen fertilizer etc.) and elemental Sulphur (e.g., used as a drug or in making gunpowder and fireworks).
References
- https://www.sciencedirect.com/topics/chemistry/nitrogen-compound
- https://opentextbc.ca/chemistry/chapter/18-7-occurrence-preparation-and-properties-of-nitrogen/
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/sulfur-compounds
- https://www.rsc.org/periodic-table/element/16/sulfur
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_16%3A_The_Oxygen_Family_(The_Chalcogens)/Z016_Chemistry_of_Sulfur_(Z16).





