Table of Contents
Key Facts & Summary
- Elements are composed of one type of atom. An element cannot be decomposed into simple substances
- Compounds can be decomposed into elements
- Compounds are composed of two or more types of atoms
- A Chemical formula is a representation of the number of atoms of each element that makes a compound.
- The different types of formulas are molecular, empirical, structure, and condensed chemical formulas.
- A chemical equation represents the processes that occur during a chemical reaction, using numbers and symbols
- A chemical reaction needs to be balanced.
Compounds
When two or more elements are chemically joined, a compound is formed. Water, salt, and sugar are examples of compounds. When the elements are joined, the atoms lose their individual properties and acquire different properties.
Some compounds are made of molecules (linked collection of atoms like H2O) and some are made of ions (positive and negative charged atoms) NaCl is made of a number Na+ and Cl– ions in a three-dimensional array but NOT NaCl molecules.
A chemical formula is used a quick way to show the composition of compounds. Letters, numbers, and symbols are used to represent elements and the number of elements in each compound.
Chemical formulae
Each substance present in nature has a specific chemical composition, and therefore they have their own unique chemical formula.
Simply put, a chemical formula is a representation of the number of atoms of each element that makes a compound. It contains the symbols of the atoms of the elements present in the compound and the number of each element in the form of subscripts.
For example in the glucose, the sugar you use to sweetener the coffee there are 12 carbon (C) atoms, 22 hydrogen (H) atoms and 11 oxygen (O) atom, so the chemical formula is C6H12O6
Chemical formulas can be quite simple such as the formula for hydrogen, which is H, or it can be much more complicated, such as the formula for ethanol, a particular alcohol, CH3CH2OH.
Also, there are different types of formulas, including molecular, empirical, structure, and condensed chemical formulas.
Molecular Formula
Also known as the "true formula", the molecular formula states the actual number of atoms of the elements in a single molecule. For example, as we have seen above, the molecular formula of the sugar glucose is C6H12O6.
Empirical Formula
The empirical formula gets its name from empirical data and it represents the simplest ratio of the whole number of elements in a compound. It can be the same as the molecular formula, as it happens with the formula of water (H2O), while other times the formulae are different. For example, the empirical formula of glucose is CH2O, which is obtained by dividing all of the subscripts by the common value (6, in this case).
Structural Formula
A structural formula shows the chemical bonds, and how the atoms are arranged.
This is important information because two molecules may have the same number and type of atoms, but arranged in a different way. (Figure 1)
For example, ethanol (common alcohol people can drink) and dimethyl ether (a toxic compound) share the same molecular and empirical formulas but have a different structural formula. This means that the same atoms are arranged in a different way.
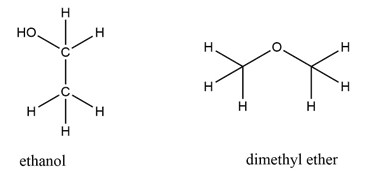
Fig.1 structural formulae of ethanol and dimethyl ether
Condensed Structural Formula
The condensed structural formula may omit the symbols for carbon and hydrogen in the structure, simply indicating the chemical bonds and formulas of functional groups. It is important because this formula indicates the position of the atoms and the functional group in the molecule structure, which is quite useful when studying organic chemistry. The written condensed formula lists the atoms in the order in which they appear in the molecular structure. For example, the molecular formula of hexane is C6H14, but its condensed formula is CH3(CH2)4CH3.
Chemical equations
A chemical equation is a way to represent the processes that occur during a chemical reaction, using numbers and symbols.
The reactants of the reactions are written on the left side of an arrow, and the products are written on the right side. The arrow usually points to the products, but on some occasions is directed in both way, indicating an equilibrium, where the chemical reaction is proceeding in both way, at the same time.
The elements in an equation are denoted using their symbols. Coefficients next to the symbols indicate the stoichiometric numbers. Subscripts are used to indicate the number of atoms of an element present in a chemical species.
Methane + Oxygen → Carbon Dioxide + Water
CH4 + 2O2 → CO2 + H2O
The participants in the chemical reactions are element symbols. In this reaction, C is carbon, H is hydrogen, and O is oxygen. Usually, you will need to know the element symbols, in order to understand the equations.
The reactants are written on the left side of the arrow, and in this reaction are CH4 + O2
The products are written on the right side of the arrow, and in this reaction are CO2 + H2O
The arrow between the reactants and products should point from left to right or, if the reaction is proceeding both ways, point in both directions (this is common). If your arrow points from right to left, it's a good idea to re-write the equation the conventional way.
Balancing Mass and Charge
Chemical reactions need to be balanced. An unbalanced equation lists the reactants and products, but not the ratio between them. A balanced chemical equation has the same number and types of atoms on both sides of the arrow. If ions are present, the sum of the positive and negative charges on both sides of the arrow is also the same.
Dissolve table salt is a chemical change :
NaCl → Na+ + Cl-
ions form and spread out in water. Note that the sum of the charges present on the left and on the right of the arrow, is the same: this reaction is chemically balanced.
Indicating States of Matter
It's common to indicate the state of matter in a chemical equation by including parentheses and an abbreviation right after a chemical formula. Gases are indicated by (g), liquid by (l). Aqueous solution are indicated by (aq) which means that the chemical species is in water as an aqueous solution.
Read more about Formulae Equations, and Amount of Substance
Frequently Asked Questions
What do you understand by the term compound?
A compound is formed when two or more elements combine chemically to achieve stability. For example, NaCl is an ionic compound formed by the chemical combination of sodium and chloride ions.
What are the types of chemical formulas?
Different types of chemical formulas include molecular formulas, empirical formulas, and structural formulas.
What is the difference between molecular formula and empirical formula?
A molecular formula shows the exact number of atoms of each element in a molecule. In contrast, the empirical formula shows the simplest ratio of the atoms of elements in a compound. For example, the molecular formula of glucose is C6H12O6, while the empirical formula of glucose is CH2O.
What is a chemical equation?
A chemical equation represents a chemical reaction showing different elements participating in a chemical reaction and the products formed.
CH4 + 2O2 → CO2 + H2O
Further readings:
Naming and writing the compounds: http://www.limestone.k12.il.us/teachers/rhebron/Chem_HO/C04_Naming_Writing.html





