Table of Contents
Introduction to Ionization Energy
The ionization energy of an element can be defined as the energy required to remove one or more loosely bounded electrons from the isolated gaseous atom in the ground electronic state to form a positively charged ion, i.e., form a cation. In this process, energy must be absorbed to remove electrons, and therefore it is an endothermic process.
The first ionization energy can be expressed as
X(g) + energy →X+(g) + e-
Here, X can be denoted as any isolated gaseous atoms or molecule, X+ is denoted as the resultant ion, and e- is the loosely bounded electron. The energy required for this process can be expressed in kJ/mol or electron volts (eV).
First, Second, and Third Ionization Energies
The first ionization energy is the removal of one electron from an isolated gaseous atom, and the second ionization energy is the removal of an electron from a +1 positive charge cation. So, it is very difficult to remove the second outer electron is difficult because the nucleus will hold it more. Therefore, the first ionization energy is less than the second, the third, and subsequent ionization energy.
The first ionization energy is expressed by the symbol I1, and the I2 symbol stands for second ionization energy. The successive ionization energy can also be expressed as I3, I4, I5…., and so on. Therefore I1<I2<I3……...<In.
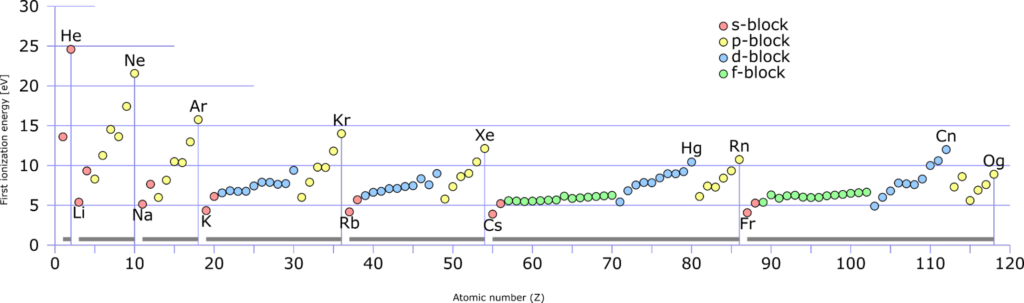
Variation of first ionization energies according to atomic number
Image Source: Wikipedia commons
Various Factors Affecting Ionization Energy
Size of an atom: When the size of an atom increases, the attraction between the nucleus and outermost electron decreases. So, removing the outermost electron from the atom can be easy, and this means that less ionization will be required for the removal of electrons from an atom.
Size of the nucleus: After the removal of the first electron, the nucleus acquires a positive charge. So, the attraction between the positive charge nucleus and the outer electron increases. Therefore, it becomes more difficult to remove a further electron from an atom. That’s why the second ionization energy is more than the first ionization energy.
Screening or Shielding effect: Outer most electron is attracted by the nucleus and repelled by the inner electrons (this is called the screening or shielding effect). When shielding increases, the attraction between the outermost electron and the nucleus decrease, and this means that less ionization energy is required.
The Periodic Table and Ionization Energy
Ionization Energy increases from left to right across the period.
When we move from the left to right in the period on a periodic table, we see that the atomic radius of an element generally decreases, so there is an effective attraction between the positively charged nuclei and negatively charged increases. Therefore, it is very difficult to remove the outermost electron easily. Alkali metal on the left side table is the minimum ionization value, and noble gas on the right side of a period is the maximum ionization value.
Ionization Energy decreases from top to bottom down in a group:
The principal quantum number of the outermost electrons increases when moving down to a group. So there, more electron shells are added when moving down in a group; therefore, the outermost electrons are so far away from the nucleus. And it is very easy to remove the outer electrons from an atom.
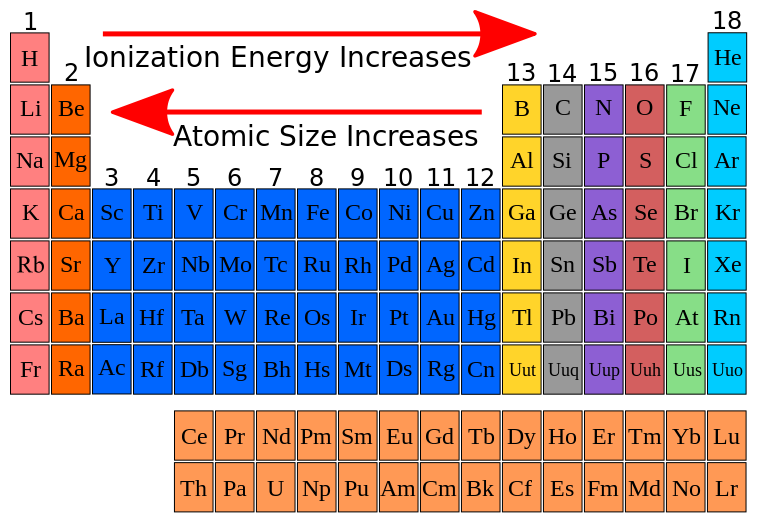
Ionization energy trend across the periodic table
Image Source: Wikipedia commons
The metallic or non-metallic character of elements in the periodic table can be explained using ionization energy. The elements with low ionization energy are the easiest to convert into positively charged ions, and these are placed at the left of the periodic table. These are metals. The transition metals are placed in the middle of the table, and as they go from left to right, the trend to ionize them keeps repeating. On the right side of the table, there are metalloids like B, C, Si, which show more covalent than ionic character, while the extreme right houses non-metals, usually gases that form negative ions.
Exceptions in the Trend of the Ionization Energy
The 1st ionization energy of beryllium is greater than that of boron, and the 1st ionization energy of nitrogen is greater than that of oxygen. This is due to Hund’s rule and the electronic configuration of these two elements. For boron, 1st ionization energy electron comes from a 2p orbital, and in the case of beryllium, it comes from a 2s orbital. On the other hand, in both oxygen and nitrogen, the electron comes from the 2p orbital, but 2p nitrogen electrons are the same spin. In contrast, one of the 2p oxygen orbitals contains a set of paired electrons.
Relation between Ionization energy, Electron Affinity, and Electronegativity:
Ionization energy is the energy that is required to remove the outermost electron from an isolated gaseous atom. So, higher ionization energy means less tendency to lose electrons; therefore, the atoms are more electronegative because they have a greater tendency to attract electrons. On the other hand, electron affinity means more energy is required to remove an electron from that atom. That’s why we see the same trends of Ionization energy, Electron affinity, and Electronegativity on the periodic table.
Ionization Energy is used in predicting Ionic and Covalent Bonds
The ionic and covalent bond formation can be determined by the difference in ionization energies and electronegativity of two reacting elements. For example,
The electronegativity of Hydrogen is 2.1; therefore two H atoms are combined and form a covalent bond.
In NaCl, there is a very large difference in ionization and electronegativity between Na and Cl atoms; as a result, they form an ionic bond.
Adiabetic and Vertical Ionization Energies
Two types of first ionization energy have been defined based on the ionization of molecules. The first type is adiabatic ionization energy, defined as the minimum amount of energy required to remove an electron from a neutral molecule. This is equal to the energy difference of the vibrational ground states of the molecule's neutral and unipositive ionic states.
The second type is the vertical ionization energy, wherein transition occurs from the vibrational ground state of the neutral molecule to the vibrational first excited state of the unipositive ion. Thus, the ionization is accompanied by vibrational excitation.
Read Electrochemistry
References:





