Table of Contents
Summary
- Mass spectrometry (MS) is an analytical technique used to identify an unknown organic compound.
- MS spectrum gives us peak mass to charge (m/z) ratio for a compound.
- Low resolution MS gives us m/z to a two decimal digits and may not be enough to distinguish between two molecules having same molecular mass (at two digit level).
- High Resolution Mass Spectrometry can offer m/z peak up to 4-5 decimal digits.
- This offers an advantage to identify two molecules to a higher level of certainty and confidence.

Mass spectrometry
Mass spectrometry is an analytical technique to identify chemical compounds, determine their chemical structure and composition by virtue of their molecular peak, fragmentation pattern, and isotopic behavior. A chemical compound is subjected to various ionization techniques and the resultants ions are characterized on the basis of their different mass–charge ratios (m/z). In the MS spectrum, we see a molecular ion peak along with peaks for different molecular fragments. These fragments are actually decomposition products when molecules are subjected to electron bombardment. Different ionization techniques have different advantages over the others and a chemist use range of these techniques to identify the chemical compound. For example, electrospray mass spectrometry (ESI) is a ‘soft’ technique where we don’t see much fragmentation and molecular ion peak is easy to figure out. While with electron impact ionization mass spectrometry gives extensive fragmentation of the molecular sample.
Low resolution MS versus High resolution MS
For general analysis or simply compound identification purposes, a low-resolution mass spectrometer is used. This gives the mass of the molecule with two decimal digits. However, there is a possibility of more than one chemical compound with the same m/z ratio. For example in a low-resolution mass spectrum for CO and N2, we see a peak at m/z 28. Another example is propane and acetaldehyde. Both molecules have m/z 44

Here, with the low-resolution MS, we cannot distinguish between the spectra of these two molecules. This is a limitation of a low-resolution mass spectrometer. For such cases, we need high resolution to separate peaks of these chemical compounds. At high-resolution MS, we get a mass peak up to 4-5 decimal digits. Now let’s examine how high-resolution MS can differentiate between propane and acetaldehyde. Let’s calculate an accurate molecular mass for both molecules using accurate exact atomic masses of carbon, hydrogen, and oxygen.
Example 1
Exact mass of 12C = 12.0000
Exact mass of 1H = 1.00783
Exact mass of 16O = 15.99491
Using these numbers, let’s calculate the mass of propane and acetaldehyde
Propane (C3H8) = (12.0000 x 3) + (1.00783 x 8) = 44.06264
Acetaldehyde (C2H4O) = (12.000 x 2) + (1.00783 x 4) + (15.99491) = 44.02623
The high resolution MS, we get m/z with 4-5 decimal digits that can identify the mass difference between propane and acetaldehyde.
Example 2
Another example is butane and propanal with the same m/z 58.

Using exact mass of atoms, let’s calculate the mass of butane and propanal
Butane (C4H10) = (12.0000 x 4) + (1.00783 x 10) = 58.0783
Propanal (C3H6O) = (12.0000 x 3) + (1.00783 x 6) + (15. 99491) = 58.04189
Here we see that accurate mass measurements are important as a means of distinguishing between chemical compounds of similar masses. This is now achieved by using high resolution mass spectrometer.
In conclusion, high-resolution mass spectrometry gives us accurate values of masses of chemical compounds to 4-5 decimal digits that help us in identifying the chemical compound to a greater certainty level and confidence.
Learn more about Mass Spectroscopy and Infrared Spectroscopy
Still limitation of HRMS!
Two isomers having the same molecular masses may not be resolved with high-resolution MS. For example:
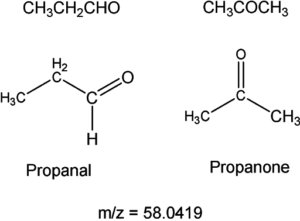
both propanal and propanone have the same molecular formula (C3H6O) and the same molecular mass. This may not be resolved with high-resolution MS and would require nuclear magnetic resonance spectroscopy as both molecules have different proton environments.
Frequently Asked Questions
What is mass spectrometry?
Mass spectrometry is a technique to identify different chemical compounds, study their structure and composition and observe isotopic behaviour.
What is the difference between HRMS and LRMS?
HRMS is more sensitive and accurate and can distinguish between two substances with similar masses. In comparison, LRMS is less sensitive and is unable to distinguish two small substances with comparable masses.
Why is high resolution important in mass spectrometry?
High resolution is required to identify and distinguish small masses. Accurate mass measurement of molecules and isotopes demands the highest possible resolution.
Does better resolution lead to better accuracy in mass spectrometry?
Yes, resolution influences the accuracy of measurement. The greater the resolution of the instrument, the greater its accuracy.
Books for further study
- Rouessac, F. and A. Rouessac. Chichester: Chemical analysis, modern instrumentation, methods and techniques. John Wiley & Sons Ltd. 2007.
- Silverstein, R. M., F.X. Webster, and D.J. Kiemle. Spectrometric identification of organic compounds. Hoboken. John wiley & sons, Inc. 2005.
- Frank A. S: Handbook of instrumental techniques in analytical chemistry. (City) Upper saddle river, 1997, Prentice Hall PTR.
- Housecroft CE, Constable EC. Chemistry. 3rd Ed. 2006. Pearson education ltd.
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





