Table of Contents
Key Facts & Summary of Haloalkanes
- Halogenoalkanes are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). They are also named haloalkanes or alkyl halides.
- They can be divided into primary, secondary and tertiary halogenoalkanes based on the number of substitutions of C which carries the halogen.
- Haloalkanes undergo substitutions reactions
- Substitution reaction can be electrophilic, nucleophilic or radical reaction.
- Halogenoalkanes (also called haloalkanes) are organic molecules that contain at least one halogen atom directly attached to the carbon skeleton.

Primary, secondary and tertiary halogenoalkanes
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group. In a secondary (2°) halogenoalkane, as the name may suggest, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different.
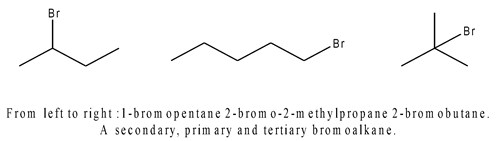
Fig1. Example of a secondary, primary and tertiary bromo-alkanes.
Another way to say this is the following: when a carbon is bonded to one other carbon, it is termed "primary" and any halogens attached to it are also termed primary. If the carbon has 2 of its bonding sites bonded to carbons, it is termed secondary, and if a halogen is attached to one of the remaining 2 sites, it is termed a secondary haloalkane.
Properties of haloalkanes:
Van der Waals dispersion forces
These type of attractions get stronger and stronger as the molecules get longer and consequently have more electrons. This is the main reason why the boiling points increase as the number of carbon atoms in the chains increases, and consequently, it takes more energy to overcome them, and so the boiling points rise. As there is an increase in number of electrons from a chloride to a bromide to a iodide (for a set number of carbons), we can see an increase of the dispersion forces and therefore an increase in boiling point. For instance, iodomethane will have a higher boiling point than chloromethane.
van der Waals dipole-dipole attractions
The carbon-halogen bonds are polar, because the electron pair is pulled closer to the halogen atom than the carbon. This is because the halogens are more electronegative than carbon. Iodine is the exception in all this, as it has the same electronegativity of carbon, therefore the bond C-I is not polar.
The electronegativity values are:
C 2.5
F 4.0
Cl 3.0
Br 2.8
I 2.5
Reactivity
From the data above, we can notice that bond strength falls as you go from C-F to C-I, and notice how much stronger the carbon-fluorine bond is than the rest.
The carbon-halogen bond, or the C-X bond, has got to be broken in order for anything to react with the halogenoalkanes. Because that gets easier as we go from fluoride to chloride to bromide to iodide, the compounds get more reactive in that order. Therefore, we can conclude that Iodoalkanes are the most reactive and fluoroalkanes are the least.
Substitution reactions.
Substitution reactions are one of the important set of reactions of halogenoalkanes involves replacing the halogen by something else. These reactions involve either:
-the C-X bond breaking to give positive and negative ions. The C+ then reacts with something either fully or slightly negatively charged.
- an anion or a cation attracted to the slightly positive or negative carbon atom and pushing off the halogen atom.
The strength of the bonds which have to be broken governs the reactivity of this compound. If is difficult to break a carbon-fluorine bond, but easy to break a carbon-iodine one.
Substitution reactions are then divided into three general classes, depending on the type of atom or group that acts as the substituent:
General formula of a substitution reaction
R-X + Nu- R-Nu + X-
where Nu stands for nucleophile and X for the halogen
1- nucleophilic substitution: the substituent is electron-rich and provides the electron pair for bonding with the substrate. Examples of nucleophilic reagents are the halogen anions (Cl-, Br-, I-), ammonia (NH3), the OH- , the alkoxy group (RO−), the cyano group and so on.
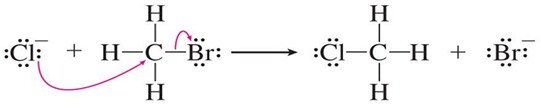
Scheme1. Example of nucleophilic substitution. http://slideplayer.com/slide/5256908/16/images/49/Nucleophilic+Substitution+Reaction.jpg
Nucleophilic substitution proceeds via 2 different mechanisms, depending on whether the haloalkane is primary or tertiary. The mechanism followed by secondary haloalkanes is thought to be a mixture of the other two.
The mechanisms followed by the primary haloalkanes is called SN2 or bimolecular nucleophilic substitution:
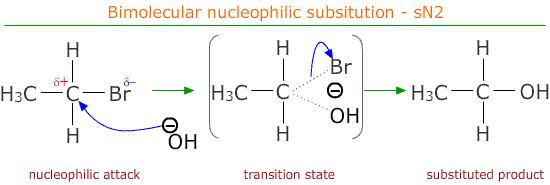
As shown in the scheme above, the nucleophile, OH- in our case. attacks the partially positive carbon that is attached to the halogen atom. This goes through a temporary high energy transition state in which the carbon atom is associated with the incoming nucleophile as well as the outgoing halide ion.
On the other hand, the mechanism followed by tertiary haloalkanes is called SN1 and a positive charge is formed, called carbocation:
The tertiary carbonium ion formed by loss of a halide ion from the halogenoalkane is sufficiently stable to exist independently. The tertiary haloalkane is in equilibrium with this carbonium ion. The mechanisms iareformed in the scheme below:
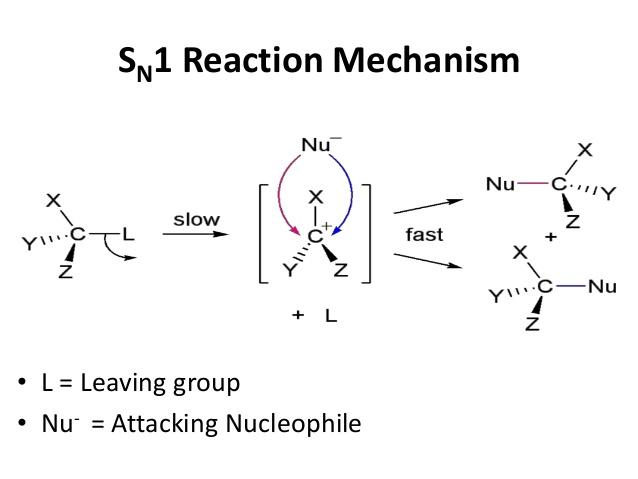
The rate of this type of reaction depends of two factors, the identity of the halogen and the nature of the halogenoalkane. The type of halogen determines the bond strength between the carbon and the halogen Primary halogenoalkanes tend to react via the SN2 mechanism which is slower (as it requires two steps). Consequently the order of reactivity is 3º > 2º > 1º
2- electrophilic substitution: the substituent is deficient in electrons, and the electron pair for bonding with the substrate comes from the substrate itself. Examples of electrophilic species are the hydrogen halides (HCl, HBr, HI), the nitronium ion (NO2+), and sulfur trioxide (SO3).
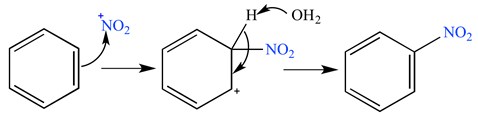
Scheme2. Example of electrophilic substitution reaction
3- radical substitution: Involve the reactions of free radicals with suitable substrates. Examples of radical reagents are the halogen radicals and oxygen-containing species derived from peroxy compounds.
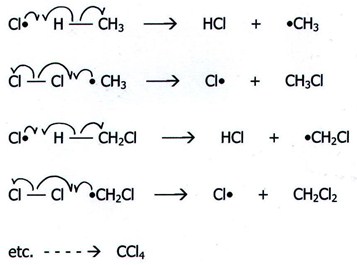
Scheme3 example of a free radical substitution reaction.
Frequently Asked Questions
What are halogen alkanes?
Halogen alkanes are chemical compounds formed by substituting one or more hydrogen atoms of the alkanes with halogens. For example, Ethyl bromide CH3CH2Br is formed by substituting one hydrogen atom of Ethane CH3CH3 with bromine.
What are the principal reactions of halogen alkanes?
Halogen alkanes mainly undergo substitution reactions. In substitution rections, one or more halogen atoms of halogen alkanes are replaced by other atoms.
What is the nucleophilic substitution reaction of halogen alkanes?
In a nucleophilic substitution reaction, a nucleophile attacks the functional carbon atom of the halogen alkane and breaks the C-X bond.
R-X + Nu- → R-Nu + X-
Are halogen alkanes polar or non-polar?
Due to the electronegativity difference between halogens and functional carbon atoms, halogen alkanes are polar compounds.
Further readings:
http://alevelchem.com/aqa_a_level_chemistry/unit3.2/sub3208/02.html





