Table of Contents
Key Facts & Summary
- Halogen have very high electronegativities
- They have seven valence electrons (one short of a stable octet)
- They are highly reactive, therefore toxics
- The halogens are Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I) and Astatine (At)
- Down the group, atom size increases.
Halogens are a group of elements on the periodic table found in group 17. They are non-metals, and the term "halogen" means "salt-former" because halogens react with metals to produce many important salts. Let's think about the chlorine ion, which is usually obtained from table salt (NaCl).
Fig1: Periodic table of the elements showing the group 17: the halogens family.https://byjus.com/jee/faq-halogens-jee/
All halogens have 7 electrons in their outer shells, giving them an oxidation number of -1. These seven outermost electrons orbit in two different kinds of orbitals designated s (with two electrons) and p (with five). Therefore, a halogen atom could get one more electron (and hold it in a p orbital), which will result in a halide ion with the same configuration than that of the noble gas next to it in the periodic table. (remember the octet rule). This is the main reason why the halogens have the tendency to acquire an additional electron making them strong oxidizers.
The halogens exist, at room temperature, in all three states of matter:
- Solid- Iodine, Astatine
- Liquid- Bromine
- Gas- Fluorine, Chlorine
but halogens are so reactive that they do not occur as free elements in nature
Properties of the Halogens
As mentioned above, these reactive nonmetals have seven valence electrons. Halogens range from solid (I2) to liquid (Br2) to gaseous (F2 and Cl2) at room temperature. As pure elements, they form diatomic molecules with atoms joined by non-polar covalent bonds.
They all have very high electronegativity. Fluorine has the highest electronegativity of all elements, and they all form negatively charged ions (H- , F-, Cl-, Br-, I-, and At-).
There is, however, a progressive change in properties from fluorine through chlorine, bromine, and iodine to astatine.
Some of the properties of this group of elements are shown in figure 2 below
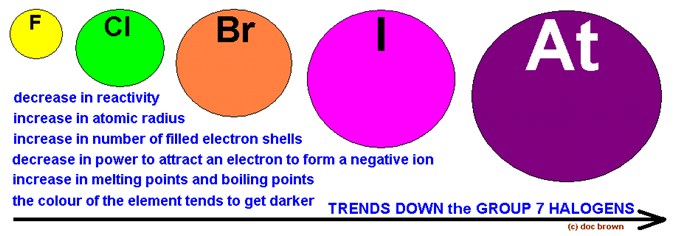
Fig 2. Scheme representing the trends of the halogens' properties in group 7
http://www.docbrown.info/page03/The_Halogens.htm
There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the melting point, boiling point, the radius of the corresponding halide ion, and the density of the element.
On the other hand, there is a regular decrease in the first ionization energy as we go down the column. As a result, there is a regular decrease in the oxidizing strength of the halogens from fluorine to iodine.
Oxidizing strength of F2 > Cl2 > Br2 > I2
This trend is inverted when we talk about the reducing strength of the corresponding halides.
reducing strength I- > Br- > Cl- > F-
Here some example of oxidation reactions:
Fluorine oxidizes water to oxygen
2F2+2H2O→4HF+O2
chlorine can oxidize bromide to bromine:
Cl2+2Br−→2Cl−+Br2
The halogen elements all form compounds with hydrogen, the hydrogen halides which are:
- hydrogen fluoride (HF),
- hydrogen chloride (HCl),
- hydrogen bromide (HBr),
- hydrogen iodide (HI),
- hydrogen astatide (HAt).
The energy of the hydrogen-halogen bond increases strongly from iodide to fluoride.
When in aqueous solution, the hydrogen halides are known as hydrohalic acids.
The names of these acids are as follows:
- hydrofluoric acid,
- hydrochloric acid,
- hydrobromic acid,
- hydroiodic acid
All of these acids are dangerous and must be handled with great care.
The halogens
Fluorine
F2 is a highly toxic, colourless gas, and it represents the most reactive element known. As an example of its reactivity, asbestos, water, and silicon burst into flame in its presence and it is difficult to find a container in which it can be stored safely.
As fluorine acts also as a powerful oxidizing agent, (it raises the oxidation state, or oxidation number, of other elements) it is handled in equipment built out of certain alloys of copper and nickel. It still reacts with these alloys, but it forms a layer of a fluoride on the surface that protects the metal from further reaction.
Fluorine is used in the manufacture of Teflon, or to make the freons used in refrigerators.
Fluorine exhibits the oxidation states of −1 (F− ion) and +1 (hypofluorous acid).
Chlorine
Cl2 is a highly toxic gas as well, and a very strong oxidizing agent, which is used commercially as a bleaching agent and as a disinfectant. It is strong enough to oxidize dyes and bleaching out colours, and strong enough to destroy bacteria. From here its use as a germicide. Large quantities of chlorine are used each year as water purifying agent or to make solvents such as carbon tetrachloride (CCl4, quite toxic), chloroform (CHCl3), dichloroethylene (C2H2Cl2), and trichloroethylene (C2HCl3), mainly used in synthetic chemistry laboratories.
Bromine
Br2 is a reddish-orange liquid, and its name comes from the Greek stem bromos, "stench." Bromine is used to prepare fire-extinguishing agents, sedatives and insecticides. It can give off suffocating vapours, corrode to the skin, and may cause severe gastroenteritis if ingested.
Iodine
I2 is an intensely coloured solid with an almost metallic luster. As its molecular weight is quite light, this solid is relatively volatile, and it sublimes when heated to form a violet-coloured gas. Iodine has been used as a disinfectant in "tincture of iodine." In organic chemistry, Iodine compounds are used as catalysts, drugs, and dyes.
The principal oxidation states of chlorine, bromine, and iodine are −1, +1, +3, +5, and +7
Astatine
As2 s the last of the known halogens and it is a synthetic element. In fact, it was synthesized in 1940 by a group of scientist in California. Astatine is a radioactive element and its most stable isotope is astatine 210 which has a half-life of 8 hours.
Not much is known about the chemical properties of astatine but it is expected to react like the other halogen.
Read more about Halogenalkanes
Frequently Asked Questions
What are halogens?
The elements of group VIIa of the periodic table are called halogens. They include fluorine, chlorine, bromine, iodine, and astatine.
What are the properties of halogens?
They combine with hydrogen to form strong acids. They form salts with metals. They have seven electrons in their outermost shell and are electronegative elements. They are highly reactive.
Do halogens conduct electricity?
Halogens are non-metals and don't conduct electricity.
Which halogen is synthetic?
Astatine (At) is a synthetic radioactive halogen synthesized by a group of scientists in California in 1940.
Further readings
http://www.chemicalelements.com/groups/halogens.html
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/group7.php
http://www.docbrown.info/page03/The_Halogens.htm





