Table of Contents
Displacement reaction is the type of chemical reaction in which the atom of the more reactive element(s) displaces the atom of the less reactive element(s) from its compound. Displacement reaction occurs in both metals and nonmetals. It is also called a replacement reaction or substitution reaction.
Imagine two people having one basket each to carry apples. The first person has space available for three apples to fill his basket completely, and the second person’s basket is getting overloaded with three apples. So, they both come together to form a stable and balanced situation for both of them by giving the extra three apples to the first person. Similarly, two elements exchange their places in their compounds to form a better and more stable compound.
The displacement reaction can be classified as:
- Single displacement reaction
- Double displacement reaction
Single displacement reaction
In this type of displacement reaction, one element that is in the form of a free element replaces the other element from its compound to make a new element and a compound. In this reaction, there is generally one free element and one compound instead of both being compounds.
Example:
2 HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)
This is the single displacement reaction because Zn is a free element, and HCl, which is a compound, forms a new element after a reaction, where “Zn” replaces the “H” from compound “HCl” and forms a new compound ZnCl2.
In general, the single displacement reaction occurs, as shown below:
A + B-C → A-C + B
This reaction occurred only because the element “A” is more reactive than B; thus, after this reaction, a more stable compound A-C is formed. In this reaction, A and B have to be either two different metals or halogens. If they are different metals, then “C” will become an anion, and if both A and B are halogens, then “C” will be a cation. It must be observed that hydrogen acts as a cation in this type of reaction; hence it can be considered a metal.
As said above, to replace an element from its compound and this single displacement reaction to occur, the element must be more reactive. Otherwise, the reaction is not possible.
Example:
CaI2(s) + Cl2(g) → CaCl2(s) + I2(s)
This reaction will occur because Chlorine, i.e. “Cl” is more reactive than iodine “I”. These are both Halogens, and the rule says “the halogens that are on the top on the periodic table in their group can replace the halogens below them, but the vice-versa is not possible.So, CaF2(s) + Br2(ℓ) → CaBr2(s) + F2(g) is not possible as “Br” is placed below Fluorine in the periodic table.
Periodic table with F and Br in 17th group
Referring to the above reaction A + B-C → A-C + B, as the elements A and B have free state nature, every single displacement reaction can also be called an oxidation-reduction reaction. This is because the important part of the reaction is the jumping of electrons from one reactant to the other. With A and B as metals, element A will always be oxidized, and element B will always undergo reduction. However, when they are halogens, then the situation will be exactly the opposite. Element A will undergo reduction, and B will be oxidised.
Types of single displacement reactions
Single displacement reactions can also be classified as the electrophilic reaction and nucleophilic reactions, on the basis of the reagent that has been used in it, considering that an electrophile or nucleophile has brought the displacement or substitution. Also, on the basis of the reactive intermediate involved in the reaction, whether it is a carbocation, carbanion or a free radical.
Nucleophiles are chemical elements that donate their pair of electrons to form a chemical bond. Any molecule or ion that has a free pair of electrons or a pi bond to replace can be a nucleophile. These are lewis bases as they have the property of donating electrons. And not accepting it. When these nucleophiles selectively bond with or attack a positive charge or even a partially positive charge on an atom or a group of atoms, then the reaction is called a nucleophilic reaction. After this reaction, the weaker nucleophile gets replaced, making it a leaving group, and the remaining positive charge or partially positive charge becomes an electrophile.
The entity of molecules containing the electrophile and leaving group formed after the above reaction is known as the substrate.
Example: Hydrolysis of alkyl bromide is a nucleophilic type of single substitution reaction.
R-Br + OH- → R-OH + Br-
Here, the base OH- is the attacking nucleophile, and the Br- becomes the leaving group.
Mechanisms of nucleophilic substitution or nucleophilic single substitution reactions are of 2 different types:
A] Unimolecular nucleophilic substitution, i.e., SN1 mechanism: It consists of two steps for the complete mechanism. In step 1, the leaving group leaves and forms a carbocation C+, and in the second step, the nucleophilic reagent forms a covalent sigma bond with the carbocation that has already been formed in step 1.
B] Bimolecular nucleophilic substitution reaction, i.e., SN2 mechanism: This involves only one step, in which the reagent’s attack and departure of the leaving group occur simultaneously. This mechanism always leads to the inversion of configuration.
Halogenation: It is the type of single displacement reaction in which halogens react with a compound and halogens take the position in the compound as well as in the free element.
Example:
CH4 + Cl-Cl → CH3Cl + HCl
Here, due to irradiation of Cl2, it breaks into 2 Cl radicals with strong nucleophilic electrons. One chlorine then breaks the C-H bond (covalent) to replace the hydrogen atom to form electrically neutral HCl, and another chlorine atom forms CH3Cl.
Electrophiles are the species that may be neutral or charged and have vacant articles that attract the electron-rich centres. These bond to the nucleophiles in a chemical reaction.
Electrophilic substitution: This is the reaction involving electrophile, which is known to be an electron pair acceptor. Electrophiles have vacant orbitals, and in an electrophilic reaction, an additional reaction occurs. In an electrophilic substitution or electrophilic addition reaction, a pi bond is broken, and two sigma bonds are formed. This reaction is mainly driven by an electron (with a positive charge), and a covalent bond is formed that has an unsaturated and electron-rich C=C bond. Then the positive charge of the electrophile gets transferred to the C-C bond, and a carbocation is formed with the formation of the C-X bond.
Then, in the next step, the positive charge of the electron reacts with an electron-rich element (which is also an anion) to form another covalent bond. This step is like the nucleophilic attack process present in the SN1 mechanism. The nature of electrophile and the positively charged intermediate element is mostly unclear and completely dependent on reactants and reaction conditions.
Double displacement reaction
We have seen in the single displacement reaction there is 1 compound, and a free element, and the free element being the more reactive, replaces an element in the compound to form one new compound and a new free element. However, in a double displacement reaction, two compounds react with each other and exchange their elements to form 2 new elements. The two reacting compounds are in their aqueous solutions and have ionic or covalent bonds. The reaction usually results in one product after the reaction gets precipitated.
A general double displacement reaction occurs like this:
A-B + C-D → A-D + C-B
So, initially, compounds A-B and C-D in their aqueous solution react to form new compounds A-D and C-B.
This simple example can help us understand the reason for this reaction. Let us consider a situation when two boys and two girls join a dance class, and they are supposed to form a couple. They randomly form a couple as A-B and C-D. However, the dancing tutor finds that A is more comfortable with D and B is more comfortable with C. So, they exchange their partners to form new couples as A-D and C-B according to their comfort, which will be better for results. Similarly, to reach a more stable state, elements form compounds with the elements which will make them more stable.
Double displacement reaction is also called metathesis and double replacement reaction. In simple words, in a double displacement reaction, cations and anions of 2 different compounds switch places with each other and form two new products.
Steps of double displacement reaction and fundamentals of the product formation
Take the reaction between Na2S and HCl.
Na2S + HCl → _____ + _____
[A] Individual ions and their charges are identified from the reactants.
In the first step, from the compound Na2S, Na is the cation and S is the anion. Generally, a cation is written first, and the anion is later. The ‘S’ has no subscript written at the bottom, which means it is one i.e. it has one atom and “Na” has subscript i.e. an integer written at the bottom, as 2. So, there are two atoms of Na for every one atom of “S” in the compound.
Now, to find the charges on the elements, we will reverse the subscripts, and the sign of the charge will be taken as positive and negative for cations and anions, respectively. Thus, Na has +1 and S has -2 charge.
In the other reactant, HCl, “H” is a cation and “Cl” is an anion, i.e., H has a positive charge, and Cl has a negative charge. Also, both “H” and “Cl” have no subscript, which means it is 1. So, we will reverse the superscript to give the magnitude of the charge on them and the sign of the charge will be given as we did for Na2S.
[B] Switching cations and anions of two different compounds.
Then, in step 2, “Na” which is the cation of the compound Na2S will get switched with “H”, the cation of the compound HCl. So, the product will be NaCl and H2S. Here, the subscript will also be switched within the elements of the compounds. Na and Cl exchange charges with each other, and similarly H gets the subscript 2.
Thus after this step, we get, Na2S + HCl → NaCl + H2S
However, the number of atoms of Na and H is not the same in the product.
[C] Balancing the reaction to equal the number of atoms.
In step 3, the number of atoms of elements will be balanced on both sides of the reaction. Because for an element, the number of atoms on the reactant side must be equal to the number of atoms on the product side. As there are two atoms of Na on the reactant side, we have to put “2” in front of NaCl on the product side, which leaves the “Cl” unbalanced. So. we put “2” in front of HCl on the reactant side.
The reaction is now balanced.
After this step, we get the reaction as written below:
Na2S + 2HCl → 2NaCl + H2S
Types of double displacement reaction
The double displacement reactions can be classified into many types, which are counter-ion exchange, alkylation, neutralisation, acid-carbonate reactions, aqueous metathesis with precipitation (precipitation reactions), and aqueous metathesis with double decomposition (double decomposition reactions). However, out of these, the two most popular are precipitation reactions and neutralisation reactions.
- Precipitation reactions take place between two aqueous ionic compounds that form a new insoluble ionic compound as a product after the reaction.
Example:
Pb(NO3)2(aq) + 2 KI(aq) → 2 KNO3(aq) + PbI2(s)
Here, lead nitrate and potassium iodide react to form potassium nitrate, which is soluble and lead iodide, which is insoluble. The lead iodide is precipitate, the solvent which is water and soluble reactants and products are supernate or supernatant. The reaction is moved forward by the formation of a precipitate.
- Neutralisation reactions are the type of double displacement reactions that involve acids and bases. When water is present as the solvent, the neutralisation reaction produces an ionic compound, i.e. salt. The presence of strong acid or base drives this reaction in the forward direction.
Example: The reaction that takes place between baking soda and vinegar in a baking soda volcano is a neutralisation reaction. This reaction moves forward and releases carbon dioxide gas which results in the fizz. The initial reaction can be written as:
NaHCO3 + CH3COOH(aq) → H2CO3 + NaCH3COO
Here, we can see that the cations have exchanged anions, but the compounds are written in a manner that might look difficult to understand and also the anion swap. The reaction can be identified as a double displacement reaction by looking at and comparing the atoms of the anions on both sides of the reaction.
The important part of predicting the product is that we must know which element can replace other elements. So, to decide which atoms and ions can replace each other, we need to understand the activity of metals and nonmetals.
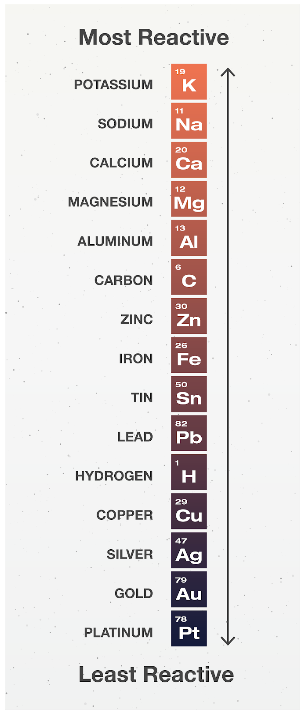
Activity series of metals and non-metals:
Atoms and ions are replaced only if it results in a better compound. Also, they get replaced with similar products; only, i.e. metals will be replaced with metals and nonmetals will be replaced with nonmetals. So, more reactive metals will replace less reactive metals, and more reactive non-metals will replace less reactive non-metals. In the periodic table, metals can be found on the left-hand side of the stairs, and non-metals can be found on the right-hand side of the stairs. In the periodic table, we can see “H” included in the metals while it is a non-metal. This is because “H” behaves like a metal in reactions.
From the activity series, we can see that Al as a metal has the ability to replace K, which is also a metal, during a displacement reaction. Similarly, for non-metals, F can replace Br as they are in the same group and F is situated above Br.
Example:
Cu + AgNO3 → Ag + Cu(NO3)2
Here, Cu has replaced Ag, and we can confirm that Cu has the capability to replace Ag as they are both situated in the 11th group and Cu is above Ag in the periodic table.
The reactivity series checks the reactivity of the elements. Based on this series, the more reactive metal will replace the less reactive metal in the displacement reaction.
Summary
- In displacement reaction, a reactant that is more reactive replaces the less reactive element from its compound to form a new compound.
- Single displacement reaction and double displacement reaction are the two types of displacement reaction.
- In a single displacement reaction, a free element replaces a part of another compound, while in double displacement reactions, both reactants are compounds, and an exchange of cations and anions takes place.
- Single displacement reactions are further classified as an electrophilic and nucleophilic reaction.
- The type of single displacement reaction that involves halogens is called halogenation.
- Precipitation reactions and neutralisation reactions are the two most popular types of double displacement reactions.
- In precipitation double displacement reaction, an insoluble precipitate is formed while in neutralisation double displacement reaction, salt is formed.
- The periodic table shows the activity series by which it can be decided which element is more reactive and which one is less reactive.
- Hydrogen is a non-metal, but it is situated on the metal side of the periodic table as, during reactions, it behaves like a metal.
Read more about collision theory
Reference
1) Wikipedia
https://en.wikipedia.org/wiki/Single_displacement_reaction
2) ThoughtCo
3) OpenStax
4) Wikimedia commons





