Table of Contents
The study of kinetics enables scientists to determine the relationship between the changes in conditions and the speed of a particular chemical reaction. One of the most important factors that affect the speed of a reaction is the reactivity of chemicals involved in that specific reaction. Furthermore, there are several variables that can also impact the rate of a reaction (concentration, temperature, catalyst, etc.).
One of such variables is considered to be a collision. Keep reading to learn more about collision theory.
Every chemical reaction requires collisions between the reactant particles (atoms and/or molecules). On the other hand, not all collisions promote the reaction to take place. Instead, if the colliding particles do not have sufficient kinetic energy or proper orientation in space, the reaction will not occur.
But what is meant by “sufficient energy” or “proper orientation”? To get a better idea of the concepts mentioned earlier, the article will provide some general definitions accompanied by corresponding examples and sample problems.
Our main goal is to define the concept of activation energy and explain why some of the collisions do not result in a reaction.
Accordingly, this article will primarily focus on the concept of reactive collision. Along with that, we will briefly overview some scenarios involving nonreactive collisions. Since collisions are associated with two major characteristics, energy, and spatial orientation, we will discuss these terms as well.
Collision Theory
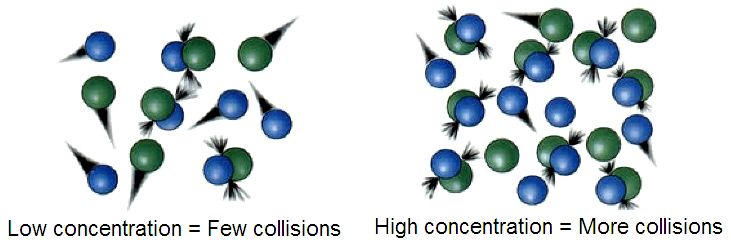
As we have already mentioned above, collisions between two or more molecules must occur in order for a chemical reaction to take place. But collision is not enough for the reactants to be transformed into products. Molecules must have enough energy, and they must collide with the correct spatial orientation.
As you may already know, there are different types of reactions, including synthesis, decomposition, displacement, and combustion. Considering the fact that collision theory focuses on the reactions involving collisions between two species, you do not have to worry much about decomposition reactions since, in such cases, a single compound is fallen apart, and there is no need to consider collisions or orientation of particles in space.
In case of reactions involving two species, collisions between the molecules are crucial for the reaction to proceed; but, only collisions of molecules with sufficient kinetic energy and relevant spatial orientation can cause a reaction. Thus, energy is needed to break the bonds within the reacting molecules, while the orientation of molecules in space plays an important role in lining up proper atoms with one another to reform the broken bonds in the proper manner.
When both criteria are satisfied, the collision is considered as a successful, effective, or reactive collision.
Considering the importance of the two factors mentioned above (energy and orientation), we should continue the further explanation of the terms and concepts to better understand the principles of collision theory.
Activation Energy
As we already mentioned earlier, particles must collide with enough energy for a reaction to occur. This minimum energy required for the reaction to proceed is defined as the activation energy.
As you may know, there are two types of reactions concerning energy release or absorption which are the following:
- Endothermic reaction – energy is absorbed in a reaction (usually in forms of heat)
- Exothermic reaction – energy is released in a reaction (usually in forms of heat)
At first glance, it might seem that since energy is released in an exothermic reaction, there should not be any need to input some energy for the reaction to occur. On the contrary, both, endothermic and exothermic chemical reactions require energy to get started.
It seems a little bit odd, right? Why does a reaction need the input of energy if the energy is consequently released?
Let’s consider the following examples of exothermic reactions occurring in our everyday lives.

Example #1 – Burning of a Candle
The burning of a candle releases energy in the form of heat; therefore, the reaction occurring during the process is exothermic. Obviously, you need to light the candle using a match or lighter. What this means is that you have to input some energy for the candle to light up. After that, the reaction has sufficient energy to proceed to the next steps, and the candle continues to burn energy.
However, a candle will never burst into flames on its own, right?
Example #2 – Lighting a Match
A typical match head is composed of sulfur mixed with different oxidizing agents. To ignite the matchstick, you should rub the match head against the special side of a matchbox. During this process, a reaction takes place and releases energy in forms of light and heat. Thus, the reaction is considered to be exothermic. Although, you still need to input some energy for the matchstick to start burning. This energy is defined via the term “activation energy.” Similarly to the previous example, as long as you provide sufficient energy for the matchstick to light up, it will continue to burn and release heat on its own.
Activation energy graph depicts the energy changes that occur during a chemical reaction.
To plot the activation energy graph, it is crucial to consider two variables:
- Number of collisions between different molecules
- Kinetic energy of the colliding molecules
The first variable is quite easy to understand since it has direct meaning – it defines the number of collisions between the particles of two species per second.
The second variable is the kinetic energy of the colliding molecules, which can be defined as the energy of the motion of the molecules.
- Endothermic Reaction – energy is absorbed. The energy of reactants is lower than the energy of products.
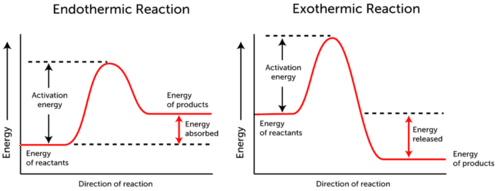
- Exothermic Reaction – energy is released. The energy of reactants is more than the energy of products.
As you can see in the two graphs above, activation energy for both, endothermic and exothermic reactions is the same.
Since endothermic reaction cannot produce as much energy as it needs to consume for the reaction to occur, the energy is absorbed; therefore, the energy of the products is higher than the energy of the reactants.
On the contrary, exothermic reactions produce more energy than they use as activation energy. As a result, the energy is released, and the products have lower energy than the reactants.
As a conclusion, it can be stated that even if reactant molecules rapidly collide with each other, it is essential for the molecules to have sufficient energy for the reaction to occur.
Spatial Orientation
According to the collision theory, not all collisions are successful, even if the reactant molecules have sufficient activation energy. The molecules must also collide the right way around.
So, the second aspect that influences reactive collisions is considered to be spatial orientation (orientation of molecules in space).
What is meant by the “proper” orientation of molecules in a reaction?
When two molecules collide, both molecules must be situated in the way that it is easy for them to react. To fully understand the concept, it is important to highlight the mechanism of the reaction.
Let’s consider the reaction between propylene (or propene) and hydrogen bromide as an example:

As you can see above, the reaction proceeds in 2 steps.
Step 1: the double bond is broken
Step 2: bromine is attached
In this reaction, the molecular orientations of HBr and C3H6 play an important role since is one of them was situated in another manner, it would not have been possible for the H atom to approach the propylene molecule.
This is true for every reaction. Each molecule must have the proper orientation in space for the reaction to take place. Correct orientation ensures that the molecules are situated in the way that it is relatively easy for them to collide and react.
We can conclude that the favourable orientation of reactant molecules is as essential as sufficient activation energy.
Summary
Terms and concepts defined throughout the article are summarized in the table provided below:
| Collision Theory | Explains how the particles should interact or with each other to cause a reaction. According to the Collision Theory, two criteria must be met in order for a chemical reaction to take place: Molecules must collide with sufficient activation energy to break and re-form existing bonds; Molecules must collide with favourable spatial orientation. |
| Successful Collision (also referred to as effective, or reactive collision) | A collision of molecules with sufficient activation energy and proper orientation in space that results in a chemical reaction. |
| Activation Energy | The minimum energy that is required for the reaction to occur. The input of energy is needed in case of endothermic as well as exothermic reactions. |
| Endothermic Reaction | Energy (heat) is absorbed; The energy of reactants is LOWER than the energy of products; Can not produce as much energy as it consumes during the reaction. |
| Exothermic Reaction | Energy (heat) is released; The energy of reactants is HIGHER than the energy of products; Can produce more energy than it consumes during the reaction. |
| Molecular Orientation | Spatial orientation of molecules. |
Three different scenarios are represented in the picture attached below:
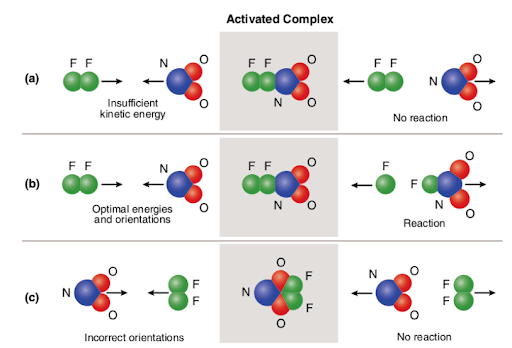
The picture summarizes the concept of collision theory utilizing two reactant molecules (fluorine (F2) and molecular nitrogen dioxide (NO2)) and three different cases to form nitrogen oxyfluoride.
In the case of (a), the reactant molecules have proper spatial orientation but do not have sufficient activation energy; Therefore, the reaction does not occur due to the fact that there is not enough energy to break bonds. So, the collision between the two molecules is considered to be nonreactive.
In the case of (b), the reactant molecules have both sufficient activation energy and proper orientation in space. Due to the optimal characteristics of the molecules, the reaction proceeds and nitrogen oxyfluoride is formed. So, the collision between the two molecules is considered to be reactive.
In the case of (c), the reactant molecules have sufficient activation energy but are not properly situated in space; Therefore, the reaction does not take place since it is not possible for the molecules to reach one another in the way that breaks bonds. So, similarly to the first case, the collision between the two molecules is considered to be nonreactive.
Frequently Asked Questions
What does collision theory explain?
According to collision theory, a chemical reaction occurs when two molecules collide with enough energy and proper orientation.
What are the three parts of collision theory?
According to collision theory, three conditions must be satisfied for a chemical reaction:
Molecules must collide with each other
Colliding molecules must have enough energy
Colliding molecules must have the proper orientation to react.
Why is the energy required for a reaction to take place?
Every reaction needs some amount of energy to initiate effective collisions. This minimum amount of energy is called activation energy and must be supplied before a reaction begins.
What is meant by proper orientation in a reaction?
Proper orientation means that when two molecules react, they must be situated so that it's easy for them to react.
References:
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





