Table of Contents
Introduction to Combustion Analysis
As we know from our day-to-day experience, a fire burns materials into smoke and ashes. This transformation is widely known as the very fundamental form of combustion. A more refined understanding of combustion comes from a chemical perspective that views the process as the mixing and exothermic reaction of a fuel and an oxidizer. From the ancient techniques of mud ovens to modern automobiles, combustion has found the most important position in human progress. The ability of modern analytical techniques to quantify the elements present in materials is on a large scale owing to combustion analysis.
Combustion analysis is the technique of determining compounds' elemental composition (empirical formula) by combusting the samples into simple and small molecules from which the information about the original composition can be extracted. Four elements, carbon, hydrogen, nitrogen, and sulfur, are combusted in the presence of oxygen and converted to carbon dioxide, water vapor, nitrogen dioxide or nitric oxide, and sulfur dioxide. The ratio of these gases provides information about the ratio in which these elements are present in a sample and consequently serves to find the empirical formula of a compound. The notable uses of combustion analysis are in the fields of measuring total nitrogen in food or feed to determine protein percentage, measuring sulfur in petroleum products, or measuring total organic carbon (TOC) in water.
Choice of flame in performing combustion is one of the most crucial aspects determining the outcome of the products to be analyzed. The amount of oxygen in the combustion system can alter the flame and its appearance. A flame with no oxygen tends to have a very turbulent flow, while a flame with an excess of oxygen tends to have a laminar flow. For steady and uniform combustion, clean fuel and an abundance of oxygen are necessary.

Combustion Train
A combustion train is an analytical instrument to determine the elemental components in a chemical compound. The information about the elemental composition derived from this can be used to arrive at a chemical formula. This instrument allows C and H through multiple steps.
In the first step, the sample is combusted at high temperatures with Copper(II) oxide (oxidizing agent). The next step is the collection of the resultant gas using a hygroscopic agent (magnesium perchlorate or calcium chloride) that traps the water vapor. The third step utilizes a strong base to collect the excess gas in a strong base (like potassium hydroxide) to trap the carbon dioxide. A known amount of the sample allows the calculation of the empirical formula by estimating the amounts of water and carbon dioxide.
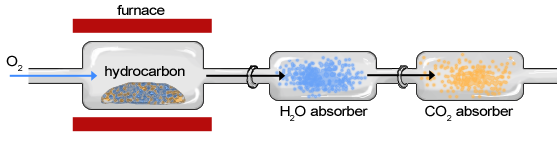
The Basic Design of the Combustion Analyzer
A furnace houses the sample compound so that it is burned in the presence of oxygen. As the resultant gases pass through the water vapor absorber and carbon dioxide absorber, the weight of each chamber changes, and this is the weight of the respective gases collected.
Calculations in Combustion Analysis
Hydrocarbons are compounds containing only H and C atoms. Therefore, combustion analysis provides straightforward results that accurately depict their empirical formulas.
The reaction with oxygen is as follows:
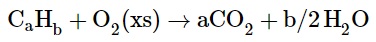
Compounds that contain other elements like O, N, and S can also be analyzed by combustion analysis. Still, the direct determination of water vapor and carbon dioxide does not give their ratios. For those, the mass of the gases like nitrogen dioxide, nitric oxide, and sulfur dioxide are determined by weighing. The final ratio of the elements is obtained by adding this data to the ratio of C and H.
For example, let us assume a compound containing carbon, hydrogen, and oxygen was burned in a combustion analysis apparatus. The 2.0714 g sample yielded 1.928 g of H2O and 4.709 g of CO2, and the molar mass of the sample was determined to be 116.16 g/mol.
In the first step of the calculation, the molar masses of water and carbon dioxide would let us determine the moles of hydrogen and carbon.
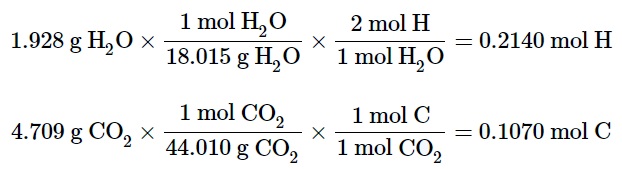
The second step is to calculate the masses of hydrogen and carbon in the original sample using the values from the first step.
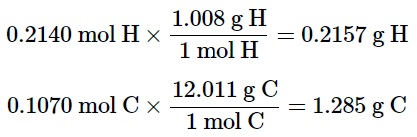
The third step is subtracting the masses of hydrogen and carbon from the sample mass, and this allows us to determine the mass of oxygen in the sample.

In the fourth step, dividing each molar amount by the smallest molar amount allows us to determine the ratio between the three elements.
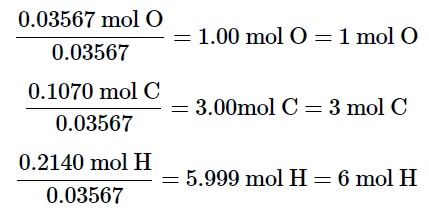
Thus, the empirical formula of the compound comes out to be C3H6O.
The molecular formula can be determined in the fifth and final step by dividing the experimental molar mass of the unknown hydrocarbon by the empirical formula weight.
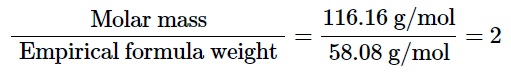
Hence, the molecular formula comes out as C6H12O2.
As it is obvious from this analysis, combustion does not provide information on how the atoms are bonded in a compound. Thus this technique has to be used in addition to other techniques for further analysis.
Modern CHNS Analyzers
In modern times, carbon, hydrogen, nitrogen, and sulfur analyzers have been developed based on the principle of the "Dumas method," using flash combustion of the sample to cause instantaneous oxidization into simple compounds. Thermal conductivity or infrared spectroscopy is used to detect oxidation products in these instruments, while chemical reagents are used to separate interferences.
Advantages of Combustion Analysis over Spectroscopic Methods
- Solid samples with C, N, H, and S at concentrations less than 1 ppm can be accurately measured by combustion analysis, which is impossible even by high-tech instruments like XFS and ICP OES.
- Inhomogeneous samples containing carbon can be easily analyzed without the need for extensive curation of the samples.
- Total organic carbon (TOC) and total inorganic carbon (TIC) can be measured in soil and water samples.
References:
- https://chem.libretexts.org/ Introduction to Combustion Analysis
- https://www.sciencedirect.com/topics/engineering/combustion-analysis
- https://www.chem.ucalgary.ca/courses/351/Carey5th/useful/cdea.html
- Image sources: https://chem.libretexts.org/





