Table of Contents
Summary
- Combustion is a chemical reaction that involves burning the organic compounds
- This chemical reaction converts organic compound into carbon dioxide and water with the release of heat energy.
- Heat of combustion is the amount of energy released when one mole of a substance is burned.
- Combustion reaction is an important process in our daily life as we use hydrocarbons as source of energy for domestic and industrial processes.
- Combustion of natural gas and gasoline combustion are typical examples of this chemical reaction.
- Keep reading for more facts about the combustion of alkanes.
Alkanes are saturated hydrocarbons with a central carbon atom attached to four other atoms (or groups). This saturation leads to relatively low reactivity of alkanes. Traditionally in old times, saturated hydrocarbons were known as paraffins meaning “little affinity”. However, these alkanes burn very rapidly. The combination of alkanes with oxygen-generating heat is known as combustion. More precisely, combustion is defined as “a chemical reaction with oxygen in which alkane is converted into carbon dioxide and water with the release of heat energy”.

Heat of Combustion or Enthalpy
A chemical reaction is a means for breaking the old bonds and forming new bonds. The breaking of old bonds requires some energy. This is called the energy of activation. The reactant molecule must acquire or possess this energy to start the chemical. In the case of combustion, slight ignition or electric spark can provide this initial energy. The heat generation in combustion reaction is because: the energy required breaking old bonds (bonds of CH4 & O2) is less than the energy released by the formation of new bonds (H2O & CO2). Hence the system releases energy in the form of heat. The heat released on complete combustion of one mole of a substance is called heat of combustion and the reaction is called Exothermic.
ΔHo = Hoproducts - Horeactants
Here H is the heat content or enthalpy of a substance in its standard state (at room temperature and at atmospheric pressure). In an exothermic reaction, the enthalpy of the product is less than starting reactants and thus reaction generates heat energy and ΔHo is negative. SI unit for heat energy & enthalpy is Kilojoule/mole (kJ/mol).

Combustion of larger hydrocarbons
Data about the heat of combustion for various saturated hydrocarbon is available suggesting that with an increasing number of carbon atoms in a molecule, the heat of combustion increases. This is obviously due to more carbon available for burning and more bonds undergoing changes. Equation 1 & 2 demonstrates this where methane, a single carbon molecule generates less heat energy compared to butane with four carbon atoms generating more heat energy.
However, the larger hydrocarbons with increasing carbon chains are harder to ignite. This is because the bigger molecules don't vaporize easily. The combustion reaction is facilitated if the oxygen and the hydrocarbon are well mixed as gases. Bigger molecules have greater Vander-Waals attractions that make it difficult for them to break away from their neighbors and turn to gas.
Colour of flame
The burning of the hydrocarbon-oxygen mixture can be complete or incomplete. The two cases depend upon the size of hydrocarbon and oxygen supply. In case of complete combustion, hydrocarbons will burn with a blue flame while yellow flame shows incomplete combustion. Also, the combustion will be less complete as the number of carbon atoms in the molecules increases. That means that the bigger the hydrocarbon, the more likely you are to get a yellow, smoky flame. Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon particles or carbon monoxide. As a simple way of thinking about it, the hydrogen in the hydrocarbon gets the first chance at the oxygen, and the carbon gets the leftover. The presence of carbon particles in a flame turns it yellow, and black carbon is often visible in the smoke.
Relative stability of hydrocarbons
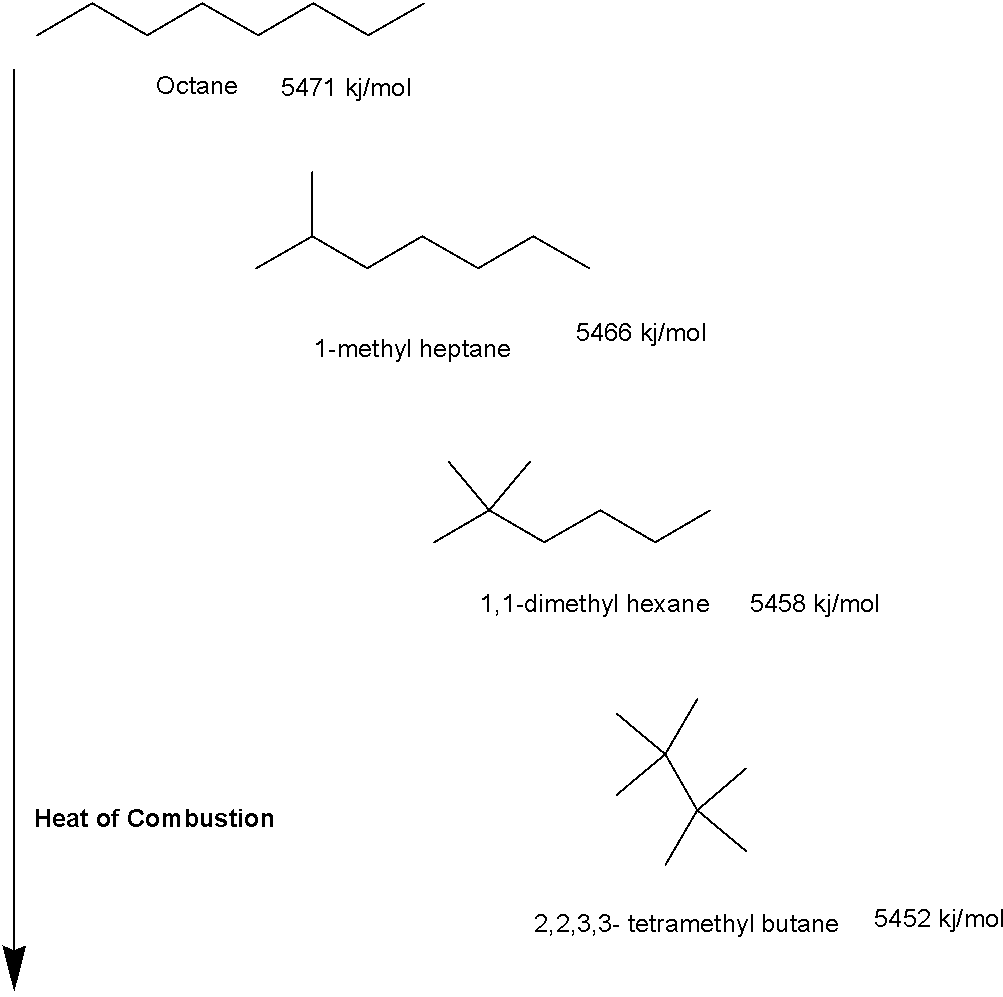
The values of heat of combustion offer an important assessment in understanding the relative stability of isomers. Different compounds with exactly the same molecular mass but different structures are called isomers. This structural difference means they have different physical and chemical properties. They might have the same number of carbon, hydrogen, or oxygen (or other elements) atoms; however, these atoms are attached in a different way or at different locations. Figure 1 shows different isomers of octane molecules. These isomers have the same molecular mass but different structures because methyl groups are attached in a different way in each molecule. The heat of combustion for each isomer of the same octane compound is slightly different. The amount of heat energy released is decreasing with increasing branching where the highly branched isomer 2,2,3,3-tetramethyl butane generates low energy. We may put this heat of combustion in a potential energy diagram. Potential energy is the exclusive energy that molecules possess for their kinetic motion. Since these isomers undergo the same combustion reaction and produce the same number of moles of water and carbon dioxide, the difference in heat generated can be translated to their different potential energy. A molecule with more potential energy is less stable while the molecule with less potential energy (or less heat of combustion generated) is more stable. Hence in the example of octane, unbranched octane is less stable than its branched isomers.
Dipole-Dipole Interaction
The extra stability of a branched molecule is due to increased intramolecular forces where more methyl groups are in the vicinity and are interacting with each other via dipole-dipole interactions. These interactions stabilize a more branched molecule compared to a straight-chain molecule where no such dipole-dipole interaction is in action.
Practical scenarios of Combustion reaction
A combustion reaction is an important feature in our everyday life. Our domestic cookers, heating homes, and engine-moving vehicles all involve the combustion of hydrocarbons. We need heat energy for our daily works and hydrocarbons are the raw material used to supply us with the required heat energy. Combustion of natural gas in our domestic cooker is a simple example of where methane gas is ignited with an electric spark or phosphorus stick. Similarly other gaseous hydrocarbons e.g. ethane, propane or butane are used for different operations.
Crude oil provides us with gasoline or petrol, kerosene, and diesel to move our vehicles. Gasoline is chemically a mixture of octane, isooctane, and other hydrocarbons used for light transport vehicles (LTV).
Internal Combustion engine
Combustion of the gasoline-air mixture (or other hydrocarbon fuel) inside the engine generates gases. These hot gases drive the piston of the engine as they expand with increasing temperature. This is called internal combustion ignition. The reaction of this reaction is highly complex and seems to follow a free radical mechanism. Octane is an eight-carbon chain molecule and its combustion is highly exothermic. However, the explosion of this reaction in the cylinder generates knocking which reduces the engine power. To overcome this problem, octane is replaced or mixed with more branched isomers e.g. isooctane. Knocking can also be solved by adding an anti-knocking agent. Tetraethyl lead [(C2H5)4Pb] has been in use for years as a popular anti-knocking agent until recently environmental concerns have been raised about increasing lead accumulations. Leaded Gasoline is a known fuel at gas stations. During combustion reaction, lead is oxidized to lead oxide and it is believed that series of chain-breaking reactions take place at the surface of these tiny lead particles hence lowering the knocking problem and improving the engine performance.
Combustion reaction for a chemical analysis
A combustion reaction is also used for the detection and quantification of different elements present in a molecule. The unknown compound is burnt under in a controlled chamber and the gases evolved are analyzed. Sodium fusion test is popular to detect other elements e.g. halogens, sulfur & nitrogen. The combustion with sodium metal converts halogens (or S & N) into their inorganic salts that can be detected in subsequent chemical tests to confirm the presence of halogen, sulfur & nitrogen.
Quantitative analysis
The amount of carbon & hydrogen can be quantified using a combustion train. This analytical tool measures the amount of carbon dioxide and water produced by burning organic compounds. The experiment is carried out in a closed chamber fitted with traps containing a strong base (e.g. sodium hydroxide) and a drying agent (e.g. magnesium perchlorate). The water formed as a result of the combustion reaction is absorbed by the drying agent while the strong base absorbs carbon dioxide. The difference in initial and final weights of collecting tubes gives an accurate amount of carbon dioxide and water produced by burning the organic compounds under study. Similarly, a nitrogen trap can also be placed. This is called elemental composition analysis. Other elements e.g. halogens can also be analyzed in a similar way.
Frequently Asked Questions
What are the products of alkane combustion?
Alkanes, upon combustion, are completely oxidized to carbon dioxide, water releasing a tremendous amount of energy.
Why is heat released in the combustion of alkanes?
Heat is released in the combustion of alkanes as the energy released during the formation of new bonds (in H2O and CO2) is greater than the energy required to break the old bonds (C-H bonds).
What is the colour of flame in alkane combustion?
The colour of the flame of alkane combustion depends upon oxygen availability. In the tremendous supply of oxygen, the flame colour is blue, while when the oxygen supply is reduced flame colour change to yellow.
What is the product of incomplete combustion of alkanes?
Incomplete combustion of alkanes produces CO2, H2O, CO, and heat.
Books for further study
- Morrison, R. T., and R. N. Boyd. "Organic chemistry 5th edition." (1987).
- Cary, A, F. Organic Chemistry, 3rd edition, (1996).
- Volhardt K, P, C. Organic Chemistry, (1987).
- Smith M, B and March, J. March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th edition (2001).





