Table of Contents
Key Information & Summary
- Aromatic compounds are unsaturated compounds having alternate double and single bonds in one or more planar rings of atoms.
- Benzene, a hexagon, is the simplest aromatic compound.
- Aromatic compounds have delocalized electrons called pi electrons that provide overall stability to these compounds.
- Criteria for aromaticity includes that the compound is planar, cyclic, has complete delocalization of pi electrons and meets Huckel’s rule.
- Nomenclature of aromatic compounds includes IUPAC names as well as common names. Many of the common names are accepted in IUPAC system as well.
Chemical reactions of aromatic compounds include electrophilic substitution, nucleophilic substitution, hydrogenation and coupling reactions.
Introduction
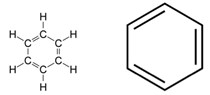
Aromatic compounds are the unsaturated compounds having one or more planar rings of atoms with alternate single and bonds. These compounds have unique stability known as aromaticity. The word “aromatic” means “odorous”. Many aromatic compounds have an odour but not all odorous compounds are aromatic. Aromatic compounds are generally non-polar and unreactive. They are immiscible with water but may be used to dissolve other non-polar compounds. Benzene is the simplest aromatic compound. It is a hexagon with alternating single and double bonds.
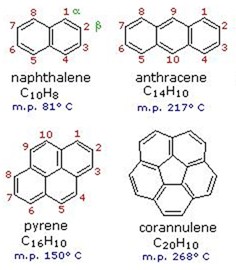
Benzene rings may fuse together to give large and complex polycyclic aromatic compounds. For example, naphthalene, anthracene, pyrene and corannulene have two, three, four and five fused benzene rings, respectively.
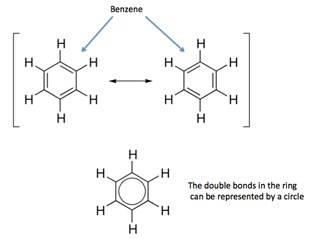
Pi electron cloud
The rings of aromatic compounds contain delocalized electrons i.e. the electrons that are not fixed to one atom and instead spread all over the ring. For instance, in benzene the electrons keep moving in the ring forming alternate double bonds. These electrons are called pi electrons. The delocalization of electrons provides greater overall stability to the aromatic compounds. These delocalized electrons can also be represented as a circle within the ring as shown below.
Read more about the Analysis of Organic Compounds
Criteria for aromaticity
A compound may be regarded as aromatic compound if it follows the following four rules:
- It is cyclic i.e. contains a ring of atoms
- It is planar i.e. all the molecules lie in same plane
- It has complete delocalization of pi electrons in the ring
- It follows Huckel’s rule (4n+2, where n=0 or any positive integer)
For calculation of Huckel’s rule, the number of pi electrons has to be calculated. Pi electrons lie in p orbitals and each sp2 hybridized atom has 1 p orbital. If an atom has three atoms attached to it and no lone pair of electrons, it is sp2 hybridized. For example, in benzene ring, there are 6 carbon atoms and each carbon atom is attached to 2 carbon and 1 hydrogen atoms (i.e. each carbon is sp2 hybridized). In this way the pi electron number of benzene is 6. Solving it for Huckel’s rule:
4n + 2 = 6
4n = 6-2
4n = 4
n = 4/4
n = 1
If n is 0 or any positive integer (1, 2, 3, …..), it means that Huckel’s rule is met and the compound is aromatic.
Nomenclature of aromatic compounds
Benzene having common substituents like Br, Cl or NO2 etc. is named as bromobenzene, chlorobenzene and nitrobenzene, respectively. Second substituent may be added to benzene at ortho (o), meta (m) or para (p) positions as shown below. The first substituent is always considered to be at para position. For example, addition of Br on ortho position of chlorobenzene will be named as o-bromochlorobenzene. In another case, addition of another Cl on ortho position of chlorobenzene will be named as o-dichlorobenzene.
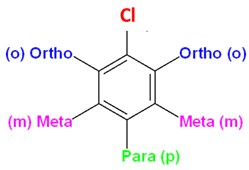
Common names of some substituted benzene derivatives can be used as a base name e.g. benzene with OH substitution is commonly called phenol. If Br is added to phenol as a substituent, it will be named as bromophenol. The figure below shows various benzene derivatives where names of the red and green coloured derivatives can be used as base names like phenol.
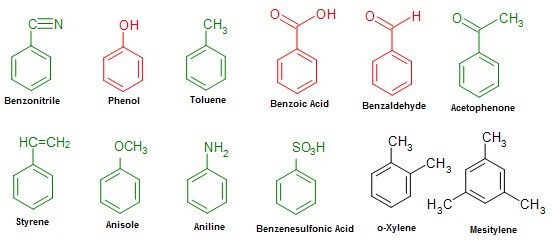
The IUPAC system accepts some of the common names for aromatic compounds like phenol, benzoic acid and benzaldehyde, it does not accept others like toluene. For instance, trinitrotoluene (named in common naming system) is named as 2-methyl-1,3,5-trinitrobenzene in IUPAC system. Also, ortho, para and meta positions are not acceptable in IUPAC nomenclature.
Chemical reactions of aromatic compounds
Aromatic compounds contain double bonds like alkenes but they cannot undergo addition reactions because the delocalization of electrons provides a stable aromatic system. However, aromatic compounds may undergo substitution (electrophilic or nucleophilic) reactions, hydrogenation and coupling reactions.
Electrophilic substitution reaction:
During electrophilic substitution reaction, the ring in aromatic compounds acts as a nucleophile. For example, when an electron deficient species reacts with benzene, it results in the addition of electrophile. This causes the formation of a high energy carbocation also known as σ complex that breaks the pi electron system. However, the delocalized electron cloud is recovered by the elimination of hydrogen. In this way, the cloud of delocalized electrons is preserved and the product is also aromatic in nature. Electrophilic substitution is also known as addition elimination-mechanism because electrophile is added followed by elimination of proton.
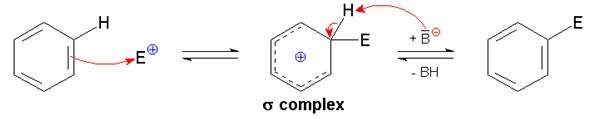
Nucleophilic substitution reaction:
During a nucleophilic substitution reaction in aromatic compounds, a nucleophile displaces a good leaving group on aromatic ring. Primarily the substituents on ortho and para positions of aromatic rings promote the nucleophilic attack while the substituents on meta position do not promote nucleophilic substitution. Attack of nucleophile breaks the pi electron system resulting in the formation of “carbanion”. The aromaticity is recovered when the leaving group “X” breaks off the ring. This substitution may also be called addition-elimination reaction.
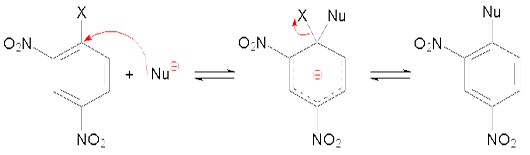
Hydrogenation:
Hydrogenation reactions convert the aromatic compounds to saturated rings. However, the stable delocalized pi electrons do not easily allow hydrogenation reactions. For example, benzene does not undergo hydrogenation in the presence of HBr or Diels-Alder. However, it gets hydrogenated in the presence of Pt catalyst in an extremely slow reaction.
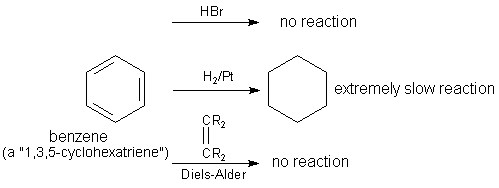
Coupling reactions:
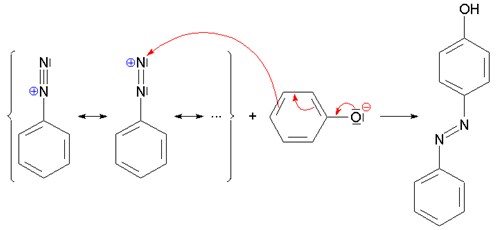
Coupling reactions result in the formation of bonds between two radicals. Examples include diazonium coupling or diazo coupling. The figure below shows diazonium coupling with phenolate in which an unsubstituted benzenediazonium cation reacts with strongly activated aromatic compound (phenolate) to form an azo compound.
Graphical Summary
Frequently Asked Questions
What are aromatic compounds?
Aromatic compounds are chemical compounds with a planar ring system having delocalized pi electrons and alternating single and double bonds such as benzene, phenol etc.
Why are aromatic compounds called aromatic?
Early discovered members of this group, such as benzene and toluene, have characteristic pleasant smell due to which this class was named aromatic compounds.
What are the criteria to classify a compound as aromatic?
The compound should have a cyclic planer ring structure with complete delocalization of pi electrons. Moreover, it should follow Huckel's rule.
What are the main reactions of aromatic compounds?
Aromatic compounds undergo both electrophilic substitution and nucleophilic substitution reactions.
References for further reading
https://crab.rutgers.edu/~alroche/Ch17.pdf
https://www.youtube.com/watch?v=r9ZV_t16x0k
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





