Table of Contents
Analysis of Organic Compounds is a practical approach towards accurate identification of formula of a compound, percentage composition of the elements present in it and the functional group(s). It involves 3 important techniques. The first one is the use of chemical tests to identify the functional groups. The second aspect is the use of high resolution mass spectrometry to identify the mass and bonding positions. The third one is the use of IR spectroscopy to confirm the functional groups and other bonding modes through “fingerprinting”.
High level research also includes the use of NMR spectroscopy for analysis of organic compounds.
Key Points in the Analysis of Organic Compounds:
- Identification of the functional group(s) is a crucial process for organic analysis.
- Mass spectrometry is the key to establishing the molecular weights of the compounds.
- IR spectroscopy helps in identification of compounds through “fingerprinting”.
Chemical Tests
Test for Unsaturation
Unsaturated compounds are those with the functional groups of carbon-carbon double or triple bonds. These can easily be detected by the following reaction:
- Bromine water test – Addition of bromine water to an unsaturated compound makes the Orange-red color of bromine disappear.
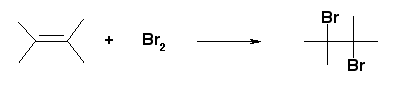
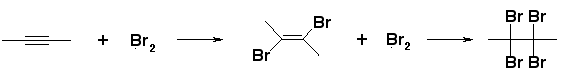
- Alkaline KMnO4 test (Bayer’s test) – The dark pink-violet color of KMnO4 disappears on addition to the unsaturated compound.
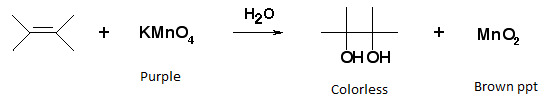
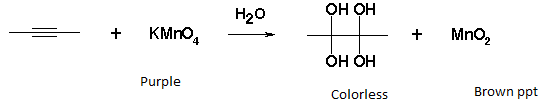
Test for alcoholic group
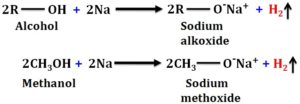
- Sodium metal test
Reaction of alcohol with metallic Na releases hydrogen gas observed as an effervescence.
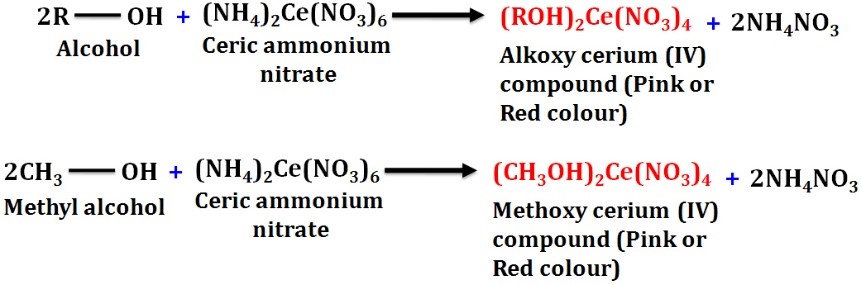
- Ceric ammonium nitrate test
Reaction of alcohol with a ceric ammonium nitrate results in the formation of red colored alkoxy cerium (IV) compound.
Tests for Aldehydes and Ketones
- DNP test for carbonyl group
The reaction of 2,4 dinitrophenylhydrazine with a ketone or an aldehyde leads to formation of yellow or orange precipitate.
![]()
- Tollen’s test to distinguish Aldehydes and Ketones.
Tollen’s reagent is ammoniacal silver nitrate. In a positive test, the diaminesilver(I) complex oxidizes the aldehyde to a carboxylate ion and in the process, is reduced to elemental Ag and aqueous ammonia. The elemental silver precipitates out of solution, occasionally onto the inner surface of the reaction vessel, giving a characteristic "silver mirror".
![]()
- Fehling’s Test to distinguish Aldehydes and Ketones.
Fehling’s solution A is an aqueous solution of copper sulphate and Fehling’s solution B is a clear solution of sodium potassium tartrate (Rochelle salt) and strong alkali (usually NaOH). The two reagents mixed in 1:1 ratio, reacts with aldehyde to produce a deep red ppt of Cu2O.

Read more about Organic Analysis
Frequently Asked Questions
How unsaturation in an organic compound can be detected?
Unsaturation in an organic compound can be detected by the bromine water test. If the compound is unsaturated, the double bond will be taken up by the bromine atoms and the colour of the bromine water disappears.
What is Tollen’s reagent?
Tollen’s reagent (ammonical silver nitrate) is prepared by making a solution of silver nitrate with ammonia. It is used to distinguish aldehydes and ketones. Aldehydes give a positive test.
Is Fehling’s test positive with ketones?
Fehling’s test is used to distinguish aldehydes and ketones. When it reacts with the aldehydes, deep red coloured precipitates of Cu2O are produced indicating the presence of aldehydes. Ketones do not give this test.
Which gas evolves when alcohol reacts with Na metal?
When alcohol reacts with a metal like Na metal, an alkoxide is formed along with the release of hydrogen (H2) gas. For example; sodium methoxide is formed when methanol reacts with sodium metal with the release of H2.
References:
- Amrita Olabs: http://amrita.olabs.edu.in/?brch=8&cnt=1&sim=141&sub=73
- http://academics.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/
Appendix/ClassificationTests/alcohol.html





