Table of Contents
Key Facts & Summary:
- The redox potential is used to describe a system's overall reducing or oxidizing capacity.
- The redox potential is measured in millivolts (mV) relative to a standard hydrogen electrode
- The Standard Hydrogen Electrode (SHE) is the 0.0V thermodynamic reference point for all potential measurements at all temperatures.
- The potential of a half-reaction measured against the SHE under standard conditions is called the standard electrode potential for that half-reaction.
- E° values do not depend on the stoichiometric coefficients for a half-reaction.
Introduction to redox and electrode potential
We already know from the previous lectures that when metals react, they give away electrons and form positive ions. This topic is all about comparing the ease with which a metal does this to form hydrated ions in solution. For example, Mg or Cu. If we want to compare the ease with which these two changes take place, let’s start writing down the two reactions:
Mg (s) → Mg2+ (aq) + 2e-
Cu (s) → Cu2+ (aq) +2e-
Usually, in chemistry, the Mg is more reactive than Cu, so the first reactions must occur much faster than the second. Let's understand why.

The hydrogen electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The Standard Hydrogen Electrode (SHE) is officially the 0.0 Volts thermodynamic reference point for all potential measurements at all temperatures. The standard is determined by the potential of a platinum electrode in the redox half reaction:
2 H+(aq) + 2e- → H2(g) at 25 °C.
To build this electrode in the lab, you need the following:
- Platinized platinum electrode
- Acid solution that has a hydrogen ion (H+) activity of 1 mol/dm3
- Hydrogen gas bubbles
- Seal to prevent interference from oxygen (and any other chemical)
- A salt bridge is used to connected the two half cells.
The reason why platinum is used for the SHE is because it is corrosion-resistant, it catalyzes the proton reduction reaction, has a high intrinsic exchange current density, and yields reproducible results. Also is used to insure a large electrochemical surface area and rapid equilibrium conditions.
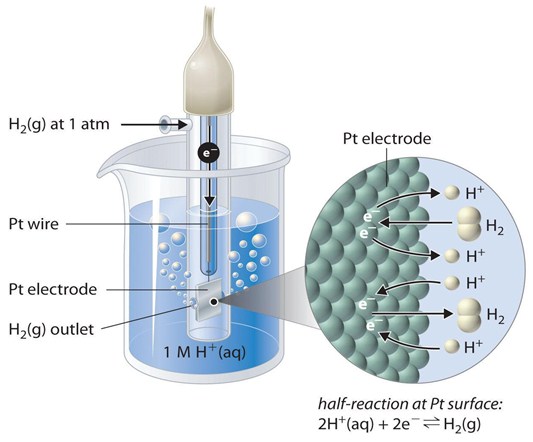
Figure 1: graphic representation of the hydrogen electrode. Important to note is the half-reaction taking place at the platinum surface. https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s23-02-standard-potentials.html
The redox reaction takes place at the platinized platinum electrode. When the electrode is dipped into the acidic solution, hydrogen gas bubbles through it. The concentration of the reduced and oxidized form is maintained, so the pressure of hydrogen gas is 1 bar or 100 kPa.
The standard hydrogen electrode is then attached to the electrode system you are investigating - for example, a piece of Zn in a solution containing Zn ions (Figure 2). The whole of this set-up is described as a cell. It is a simple system which generates and measures a voltage. Each of the two beakers are called half cells. As you can see, the half cells are connected by a salt bridge (NaCl in this case). The salt bridge is needed to complete the electrical circuit without introducing any more bits of metal into the system The ends are "stoppered", usually by cotton wool. This stops too much mixing of the contents of the salt bridge with the contents of the two beakers. The electrolyte in the salt bridge is chosen so that it doesn't react with the other chemicals.
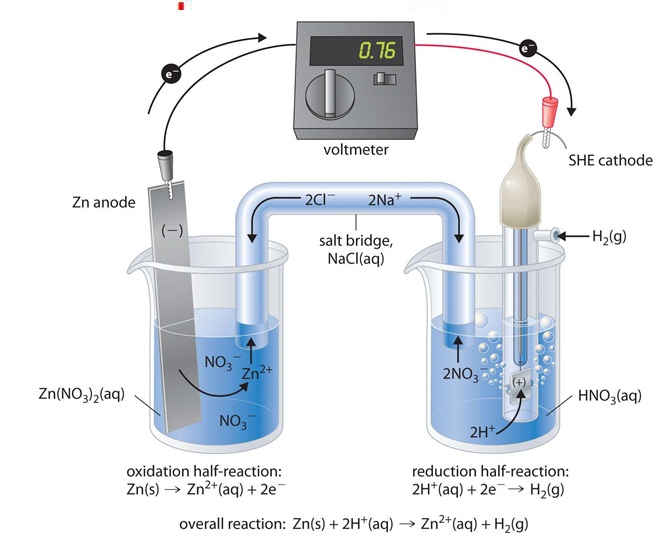
Figure 2. Graphic representation of a galvanic cell for the measurement of Zn potential. https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s23-02-standard-potentials.html
When we close the circuit, the voltmeter indicates a potential of 0.76 V. What happens is that the zinc electrode begins to dissolve to form Zn2+, and in the other cell H+ ions are reduced to H2 . Thus the hydrogen electrode is the cathode, and the zinc electrode is the anode.
The two half reaction taking place are:
cathode: 2 H + (aq)+ 2 e − → H 2 (g) E cathode ° = 0 V
anode: Ζn(s) → Ζn2 + (aq) + 2 e − E anode ° = − 0.76 V
If we combine the two half reactions
Ζn(s) + 2H + (aq) → Ζn2 + (aq) + H2 (g)
And so we can make the calculation E cell ° = E cathode ° − E anode ° = 0.76 V
A conventional way to write this cell down is the following:
Zn(s) ∣ Zn2+(aq) ∥ H+(aq, 1 M) ∣ H2(g, 1 atm) ∣ Pt(s)
The potential of a half-reaction measured against the SHE under standard conditions is called the standard electrode potential for that half-reaction ( E° )
So getting back to our first question,
Mg (s) → Mg2+ (aq) + 2e-
Cu (s) → Cu2+ (aq) +2e-
why Mg is more reactive than Cu, so why the first reaction happens faster? If we replace the Zn half cell with a Mg one we will obtain a V = 2.37 and the voltmeter would show the magnesium as the negative electrode and the hydrogen electrode as being positive. If we replace with a Cu one we will find a value of V= 0.34, which means that there will be less build-up of electrons on the copper than there is on the platinum of the hydrogen electrode, when compared with the Mg one. A change is that this time the copper is the more positive (less negative) electrode. The voltmeter will show the hydrogen electrode as the negative one and the copper electrode as positive.
It is also important to notice that standard electrode potentials for half-reactions are intensive properties and do not depend on the amount of substance involved. This is because electrical potential is the energy needed to move a charged particle in an electric field, and consequently, E° values are independent of the stoichiometric coefficients for the half-reaction.
Read more about Redox reactions
Read more about Oxidation, Reduction, and Redox Reactions
Frequently Asked Questions
What is redox potential?
Redox potential describes the overall oxidizing or reducing ability of a system. It measures how easily a system loses electrons.
What is electrode potential?
When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential.
What is the unit of redox potential?
Redox potential is measured in millivolts (mV).
What are the types of electrode potential?
Electrode potential can be oxidizing or reducing potential.
Further readings:
https://www.sciencedirect.com/topics/earth-and-planetary-sciences/redox-potential
https://chemguide.co.uk/physical/redoxeqia/introduction.html





