Table of Contents
Introduction to Nitrogen Compounds
The atmosphere contains 75.51 percent Nitrogen gas, the primary nitrogen source for industrial and commercial purposes. Due to a triple covalent bond formed between two nitrogen atoms, nitrogen is a very unreactive gas. This triple covalent bond is very strong, the bond enthalpy being 1000 kJ mol-1. So, it is very difficult for molecular nitrogen to react with O2 in the atmosphere, but it can be reactive under specific laboratory conditions.
There are various types of nitrogen-containing compounds. These are classified as mineral or organic. Inorganic nitrogen compounds include ammonium, nitrate, and nitride. On the other hand, amino acids, urea, nucleic acids, nucleotides, and proteins are examples of organic nitrogen compounds.
Inorganic Nitrogen Compounds
Ammonia
Preparation
Ammonia is chiefly prepared by Haber-Bosch process. The reaction is,
N2 (g) + 3H2 (g) ⇋ 2NH3 (g) ΔHr=-92 kJ/mol
This reaction occurs at high temperatures (400 - 550 °C), high pressure (100 - 1,000 atmospheres), and in the presence of catalysts (iron-containing iron oxide).
When ammonia is dissolved in water, it forms ammonium hydroxide, wherein a positively charged ammonium ion exists with a negatively charged hydroxide ion.
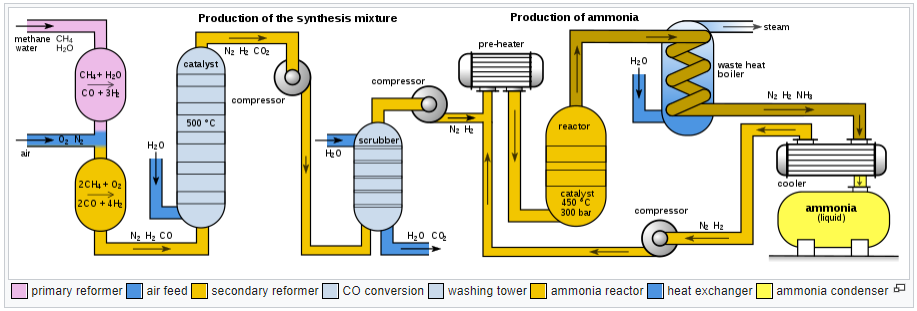
Image Source: Wikipedia commons
Uses of Ammonia
- Ammonia is mainly used as a fertilizer.
- It can also be a catalyst in many reactions, e.g., the formation of synthetic resins.
- It can be used to produce commercial explosives, e.g., TNT.
- Ammonium salt, such as urea, is a highly used fertilizer, worldwide.
Organic Nitrogen Compounds
There are four major types of nitrogen compounds. These are Amino (-C-NH2), Nitro (-C-NO2), Nitriles (-CN), and Amides ( –CONH2 ).
Amines
An amine is basically a functional group with a nitrogen atom bearing a lone pair of electrons. Amines are formed when one or more hydrogen atoms of an ammonia molecule are replaced by alkyl or aryl groups. These are the building blocks of amino acids which, in turn, are the building block of proteins.
Amines can be classified into four categories: primary amines, secondary amines, tertiary amines, and cyclic amines.
Image source: Wikipedia commons Image source: Molecules 2017, 22(10), 1691
Basicity of Amines
Bases are proton acceptors. Ammonia and amines are bases due to the presence of lone pair of electrons on nitrogen. This lone pair of electrons are donated to protons, and a coordinate bond is formed. The reaction of ammonia with H+ ions is:
NH3 + H+ → NH4+
The reaction of primary amines with H+ ions is:
CH3NH2 + H+ →CH3NH3+
The strength of ammonia, primary aliphatic amines, and aromatic amines as bases are different. Primary aliphatic amines are stronger bases when compared to ammonia. This is because the alkyl group attached to the nitrogen and Alkyl groups are electron-donating. As a result, the lone pair of electrons readily accepts the proton and a dative bond between nitrogen and the proton.
Aromatic amines are weaker bases when compared to ammonia. In aromatic amines, the lone pair of electrons on nitrogen is delocalised in the benzene ring. The lone pair of electrons is less readily available to accept protons when compared to ammonia.
The strength of bases can be summarised as primary aliphatic amines > ammonia > aromatic amines.
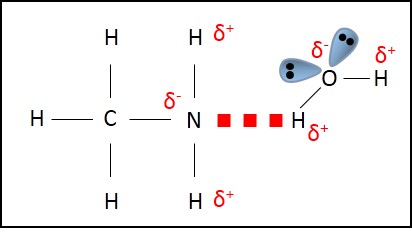
Smaller amines form hydrogen bonding with water and hence, are soluble in water.
Preparation
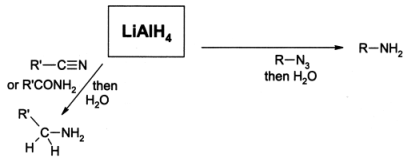
Reduction of Nitriles
Reduction of nitriles with LiAIH4 or H2 in the presence of Ni or Na in ethanol produced primary amines.
Reduction of Nitro Compounds
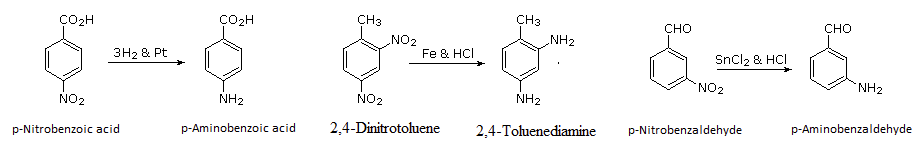
When H2 gas is passed into the nitroalkanes and nitroarenes in the presence of Ni, Pt, or Pd, it forms amines. Alternately, SnCl2 and HCl can be used for reducing nitro compounds to corresponding amines.
Uses of Amines
- Synthetic fibers, e.g., Nylon-66, can be produced from Amines.
- Amines are used for making different types of dyes, e.g., methyl orange.
- Amines can also be used in the production of emulsifiers & detergents.
- These are also used in the manufacture of various drugs and medicines.
Nitro Compounds
Nitro compounds are very special because here N atom is directly attached to the carbon atom in a -CH3 group. Thus, nitro compounds belong to one of the most important nitrogen derivatives families.
The structure of nitro compounds is made of
- Two equivalent hybrid resonating structures of the nitro group, i.e., delocalization of pi bond.
- A carbon-nitrogen bond.
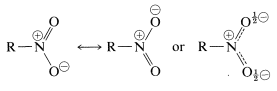
Nitro compounds are classified into three categories
- Primary nitro compounds, ex. nitroethane (CH3CH2NO2)
- Secondary nitro compounds, ex. 2-nitropropane (CH3CH3CHNO2)
- Tertiary nitro compounds, ex. 2-methyl-2-nitropropane (CH3CH3CH3CNO2)
Preparation of Nitro Compounds
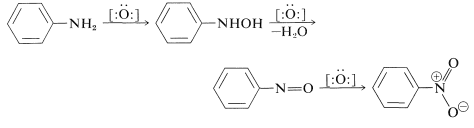
- Nitro compounds can be prepared by oxidation of 1° amines
- Nitroalkanes can be prepared by heating HNO3 gas with the alkane at around 425 °C.
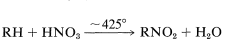
Uses of Nitro Compounds
- These can be used to prepare pharmaceuticals, detergents, and dyes.
- These are also used as propellants.
- These are also used in shoe and floor polishes.
- They are used to produce explosives like TNT.
Nitriles
Nitriles are also known as Cyano Compounds. In these compounds, the functional group is called the Cyano group (-CN). The Cyano group is formed by a sigma bond and two pi bonds, therefore it is linear in structure. On the other hand, nitrogen has a lone pair of electrons, therefore it is also polar in nature.
Preparation of Nitriles
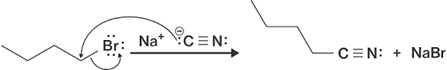
- Nitriles can be formed by SN2 reaction with bromide and NaCN.
- Dehydration of amides. Commonly, two reagents used for this purpose are phosphorus pentoxide (P2O5) and thionyl chloride (SOCl2).
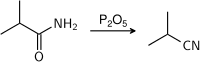
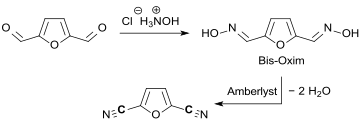
- From aldehydes and oximes using hydroxylamine salts.
Uses of Nitriles
- These are used in the manufacturing of seals, hoses, and nitrile gloves.
- These are also used to produce antidiabetic drugs.
- In organometallic chemistry, nitriles are used in carbocyanation.
Amides
Amides are compounds containing the –CONH2 group. Amides are derived from acyl chlorides, where chloride in the –COCl group is replaced by NH2.
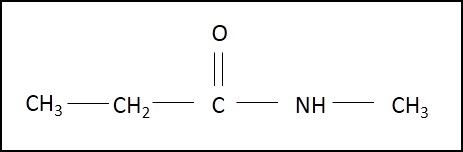
Ethanamide (primary amide) N-methylpropanamide (Secondary amide)
N, N-dimethylpropanamide and N, N,2-trimethyl propanamide (Tertiary amides)
Synthesis of Amides
- Synthesis of primary amides - Acyl chlorides react with ammonia at room temperature to form primary amides.
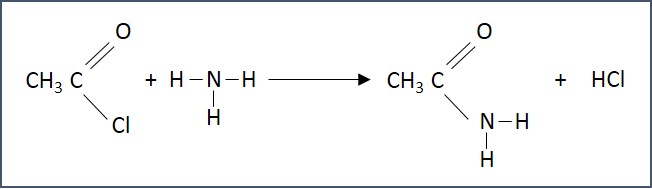
- Synthesis of secondary amides - Acyl chlorides react with primary amines at room temperature to form secondary amides.
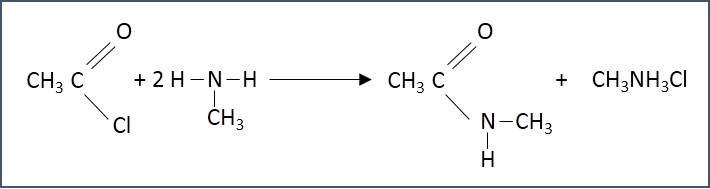
Uses of Amides
Frequently Asked Questions
What are nitrogen compounds?
Nitrogen compounds are those which contain one or more atoms of nitrogen. These compounds may be organic or inorganic. Organic compounds include proteins, nucleic acids and porphyrins etc. Inorganic nitrogenous compounds are nitrates and nitrites.
What are the uses of nitrogen compounds?
Nitric acid (HNO3) is used for many industrial purposes, including making fireworks etc. Proteins are building blocks of biological structures. Nitrates and nitrites are used as fertilizers which are an essential component of agriculture.
What is the difference between an amine and an amine?
Amines are formed when one or more hydrogen atoms of ammonia are replaced with some alkyl/aryl group (e.g., ethanolamine). While amide is formed when a carbonyl group is attached to a deprotonated molecule of ammonia (e.g., ethanamide).
Is an amine basic or acidic?
Amines are basic because the nitrogen atom has a lone pair. As we know that bases are proton acceptors, this lone pair imparts basicity to the compounds i.e., they can accept protons.





