Table of Contents
The mass number is measured by calculating the total number of neutrons and the number of protons in an atom.
Atoms
Atoms are the smallest particle of an element that combine together to form molecules that form most of the objects in the universe. A particular element is formed from only one type of atom i.e diamond is formed from carbon atoms only and gold is formed from gold atoms only.
The atoms consist of protons, neutrons and electrons.
Protons
Protons are the positively charged subatomic particles present in the atomic nuclei. They have a charge equal to that of an electron in magnitude and mass of approximately one atomic mass unit. Every element has a unique number of protons in it, and this helps in defining the atomic number of an element. The number of protons present in the nucleus of an atom is called its atomic number.
Protons are composed of 3 quarks i.e. two up quarks and one down quarks.
Note: Quarks are the fundamental constituent and elementary substance of matter that forms protons and neutrons.
Neutrons
Neutrons are the subatomic particles present in atomic nuclei like protons. These have no net charge and have mass slightly more than that of protons. These are the constituent of the nucleus of all atoms except for hydrogen.
Electrons
Electrons are the subatomic particles like protons and neutrons but located outside the nucleus and are negatively charged. These are the primary carriers of electricity. They have negligible mass as approximately 1800 electrons will equal to the mass of one proton.
Mass number
It is the number of protons plus the number of neutrons of an atom. It can also be described as the number of neutrons of that atom added to the atomic number it gives its atomic mass number.
Mass number = no. of protons + no. of neutrons
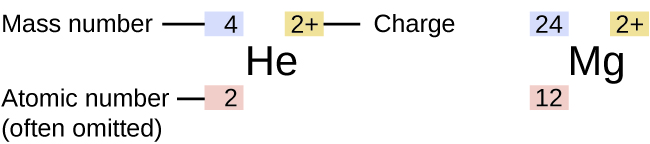
| Notation of atom Helium and Magnesium |
From the above picture,
For Helium:
Mass number = no. of protons + no. of neutrons = 4
Atomic number = no. of protons = 2
So, we can say that the number of protons present in an atom of He is two and the number of neutrons is 2. So, its mass number is 4.
Similarly, for Magnesium, the number of protons is 12, and the number of neutrons is 12. So, its mass number is 24.
Characteristics of mass number
- The mass number is written as a superscript on the left side of the symbol of the element.
- The mass number for all the elements is always a whole number. It cannot be a fraction.
Though, He-4 has a mass number 4 and atomic number 2. However, it exists in more variants as He-2, He-3, He-5, He-6, He-7, He-8, He-9, He-10. These variants have the same atomic number but a different mass number. This is because all the variants of He have the same number of protons but a different number of neutrons.
Isotope: The various forms of a particular chemical element that have the same atomic number but a different mass number are called isotopes. They have the same number of protons in their nuclei but a different number of neutrons. So, each isotope has a unique mass number.
The chemical properties of all isotopes of a particular element are the same as the number of protons and electrons is the same.
Note: Carbon, uranium and potassium isotopes occur naturally.
Some isotopes may emit electrons, neutrons and protons to have a more stable atomic structure, known as radioactive isotopes.
The mass number gives the atomic mass of the element. The mass number and atomic mass are the same but the atomic mass is mentioned as the atomic mass unit or ‘amu’. The average atomic mass depends on the mass numbers of all the isotopes.
Unlike, mass number, the atomic mass of an element can be a fraction.
Since the mass numbers of isotopes are different, we can take the weighted average of atomic masses of all the different forms of an element. This average is called relative atomic mass. The calculation of relative atomic mass also considers the abundance of the isotopes on earth.
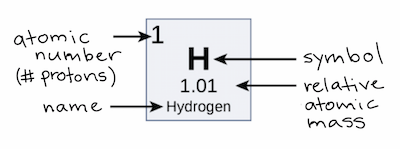
| The relative atomic mass of hydrogen |
Example:
Chlorine has a 3:1 ratio of chlorine-35 and chlorine-37. Let us say we have four atoms of chlorine.
Then the total mass will be (3 x 35) + (1 x 37) = 142.
The average mass of these four atoms will be 142/4 = 35.5
So, the relative atomic mass of chlorine is equal to 35.5
Note: The simple average of 35 and 37 would have given an average of 36. However, the weighted average is 35.5. This means that there is more number of lighter isotopes than heavier ones.
Mass spectrometry
This is an analysis method that measures the ratio of mass to charge of the ions. The abundance of each isotope is determined by using the process of mass spectrometry.
In mass spectrometry atoms and molecules are ionised using a high energy beam of the electron. Then the ions are deflected using a magnetic field, depending on their mass to charge ratios.
Stages of spectrometry
- Ionisation- It occurs by knocking off one or more electrons to give a positively charged ion. As of now, most of the mass spectrometers use positive ions.
- Acceleration- Then the ions are accelerated to give them the same kinetic energy.
- Deflection- Then the magnetic field is used to deflect the ions. The deflection of these ions depends upon their mass and charge. If the ion is lighter then it will be deflected more and if the charge on the ion is more then also it will be deflected more. The charge depends upon the number of electrons that have been knocked of in the first stage. More number of electrons are knocked off means more charge will be present on the ion.
- Detector- Then the ions reach the detector according to their masses, and computer forms the spectrum.
Read more about Mass Spectrometry
Summary
- Atoms are formed of positively charged protons, negatively charged electrons and neutral neutrons.
- The mass number is the total number of proteins and neutrons in an atom.
- Elements have isotopes with the same atomic number but the different mass number.
- The average atomic mass of isotopes is called relative atomic mass. It is calculated by mass spectrometry.
Frequently Asked Questions
What is mass number?
The mass number of an atom or isotope can be defined as the sum of the protons number and neutrons number in its nucleus.
How do you find the mass number?
The mass number of an atom of an element is the sum of the number of protons and the number of neutrons in the nucleus of an atom.
Mass number= Total no of protons + total no of neutrons
What is the difference between atomic mass number and relative atomic mass?
The mass number of an atom or isotope of an element is defined as the sum of the total number of protons and neutrons in its nucleus. In contrast, relative atomic mass is the average of atomic masses of all the isotopes of an element with their abundance compared to carbon 12.
What is the relation of mass no with atomic no?
Atomic no. is the no. of protons in the nucleus of an atom, while the mass number is the sum of proton number and neutron number. So
Atomic mass= Atomic no. + No. of neutrons
References:
- Wikipedia https://en.wikipedia.org/wiki/Mass_number
- Khan Academy https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties/mass-spectrometry/a/isotopes-and-mass-spectrometry
- Openstax https://openstax.org/books/chemistry-2e/pages/2-3-atomic-structure-and-symbolism
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





