Table of Contents
Introduction to Isotopic Abundance
Nature's elements have occurred over billions of years via nuclear reactions. As the protons and neutrons came together, the ratio of their composition in atoms took definite trends and formed the stable forms that we know today. In this route, the number of neutrons often got variable even as the proton number remained the same. This slowly began to shape the natural abundance of the elements wherein a particular form became dominant while others occupied a lesser share. These forms, known as isotopes abundance, are integral to modern scientific explorations across fields, be it biological, chemical, physical, astronomical, earth sciences, or even sports science. Over the last century, the advance in controlled nuclear reactions has enabled scientists to synthesize artificial elements and artificial isotopes of existing natural elements. The occurrence of isotopes is quite common in nature, and only 21 elements in the periodic table can get classified as “pure” elements - elements that are monotopic (only one naturally occurring isotope).
Also read: Isotopes and Mass Spectrometry
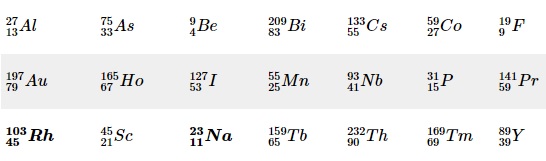
Monotopic elements Image source: https://chem.libretexts.org/
The homogenization of the elements in nature took place over billions of years of galactic events like the supernova explosions. On earth, the abundance does vary from place to place, as does the abundance of elements from planet to planet in our solar system. Nonetheless, the deviations from natural abundance on earth are less than one percent and hence often measured in parts per thousand (per mille). The sum of the percent natural abundances of all the isotopes of any given element must total 100%.
Read Isotopes and Mass Spectrometry
Role of Isotopic Abundance in the Study of Chemical Sciences
The most fundamental sheet a modern chemist must have is the periodic table of elements. In the latest release of the Periodic Table (dated 4 May 2022), IUPAC has included the most recent abridged standard atomic weight values. Atomic weights were long considered constant during the 19th and early 20th centuries. The notion started to change when Fredrick Soddy showed mesothorium (228Ra) and radium (226Ra) had identical chemical properties but different radioactive properties and atomic weights. Fredrick Soddy coined the term “isotope,” which has become the norm today. Modern mass spectrometers have come a long way in establishing that atomic weights are the weighted average of the naturally occurring isotopes. This essentially distinguishes between atomic weight, atomic mass, and mass number - the three terms that are often confused by chemistry students. As mentioned, the atomic weights are experimentally verified weights of isotopes in a particular sample of an element, averaged according to their ratios. On the other hand, the atomic mass represents the mass of the most abundant isotope of a piece, summing up the masses of the protons and neutrons present therein. The mass number of an element is a number - the sum of the number of protons and neutrons in an atom. Therefore, the study of various fields of chemistry is intricately dependent on the correct identification of elemental isotopes.
Measuring isotopic abundance has been made easy by modern mass spectrometers, instruments that allow us to measure the mass to charge ratio. An example of how this technique helps identify isotopic abundance is chlorine gas analysis. Chlorine has multiple isotopes, and when hit with a stream of ionizing electrons, the bond of Cl2 gets broken, and the electrons are stripped off, causing ions to form. These are then accelerated down the chamber until they reach a magnetic field that deflects the particles. The lighter particles are deflected more than the heavier ones, and eventually, these are separated by the magnetic field until they reach the detector. Thus, two peaks appear in the mass spectrum corresponding to 37Cl and 35Cl, the two isotopes in their natural abundance of 75% and 25%, respectively. The same process can detect the natural abundance of elements within a compound that typically follows the natural abundance in the elemental state.
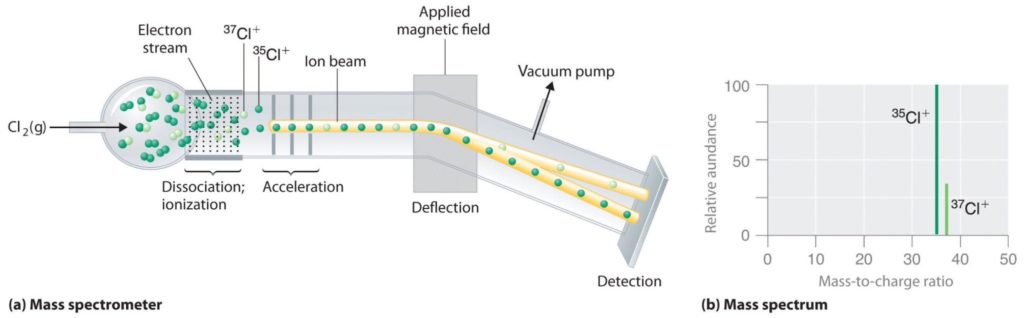
Mass spectrometry Image source: https://chem.libretexts.org/
The importance of isotopic abundance is also evident in the study of nuclear chemistry, where fission reactions are used to produce electricity using isotopes of uranium and thorium. Other uses of isotopic abundance in chemistry include the detection of radioisotopes in chemicals to understand mechanistic pathways and determine yields of reactions.
Isotopic abundance studies are also useful in chemical kinetics to determine rates of reaction and in thermochemistry of very small amounts of rare samples. Since the chemical nature of atoms is not altered by the variation in the number of neutrons, the isotope enriched compounds can be used to trace the exact position of a molecule where reactions occur.
One of the most widely used analytical procedures in chemistry is nuclear magnetic resonance (NMR) spectroscopy. This specifically depends on the isotopic abundance of the elements for which the NMR spectrum is recorded. For example, the abundance of 1H is 99.99% in nature, while deuterium is very rare and tritium is not stable under an ambient atmosphere. Utilizing this abundance, 1HNMR is used wherein 2H enriched solvents like D2O (deuterium oxide) can replace water to mask the solvent effect. In the 13C carbon NMR, since 12C is not NMR active, the peaks for each carbon in a molecule are observed distinctly, which aids in structure elucidation.
Uses of Isotopic Abundance in other Fields of Science and Technology
- Nuclear forensic applications utilize the isotopic abundance of radionuclides to characterize materials seized during smuggling or the remnants of detonation and dispersion of nuclear weapons.
- Solid scintillation analysis uses natural isotopes of Lithium.
- Biogeochemistry uses the natural abundance of sulfur to estimate its abundance in atmospheres.
- Radiocarbon dating uses carbon-14 isotope abundance and half-life to estimate the age of archeological findings.
- Cosmological dust particles contain elements of isotopic abundance different from at found on earth and hence allow us to estimate their origins.
- Earth sciences use the isotopic abundance of elements to find sources of minerals.
- In sports sciences, isotopic abundance studies help to fight doping by detecting performance-enhancing drugs via Isotope-ratio mass spectrometry (IRMS).
References
- Atomic Weights: No Longer Constants of Nature, Chem Int 33(2), 10–15 (2011), http://dx.doi.org/10.1515/ci.2011.33.2.10
- https://www.sciencedirect.com/topics/physics-and-astronomy/isotopic-abundance
- https://genecraftlabs.com/en/sports-doping-how-isotopes-help-to-fight-the-crime/
- https://chem.libretexts.org/ Isotopic Abundance and Atomic Weight





