Table of Contents
Ionisation energy is the minimum quantity or amount of energy needed by an isolated gaseous atom in its ground state to remove its valence electron or the most loosely packed/bounded electron. This leaves a cation, as an electron has been released.
The symbols of ionisation energy are IE, IP, ΔH°
The general way to express it quantitatively is as shown below:
X(g) + energy → X+(g) + e- …..(1)
Here, X is the atom or molecule that has the capability to lose electron for the ionisation and X+ is that atom or molecule when an electron ‘e-’ is removed from it.
X+(g) + energy → X2+(g) + e- …..(2)
The reaction (2) shows the further ionisation of the X+ that is received from reaction (1). The energy required in the reaction (2) will be more than the energy required in reaction (1).
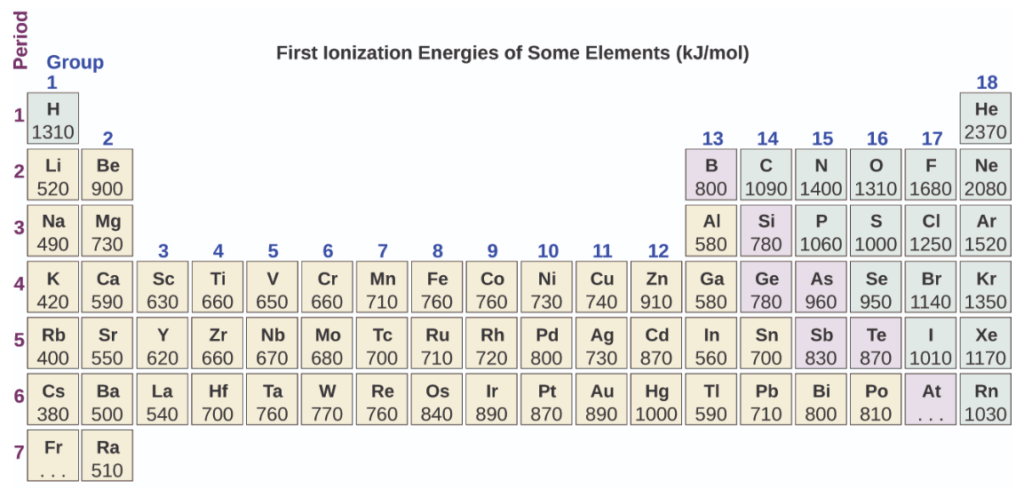
Periodic table and first ionisation energy of elements
The trend of ionisation energy
- The energy required for the ionisation i.e. for the removal of an electron increases as electrons are removed one by one. This means that the amount of energy required by the atom or molecule to release the first electron will be less than the amount of energy required to remove the second electron from that atom or molecule. Similarly, to remove the third electron, more energy will be required as compared to the amount of energy required to remove the second electron from that atom or molecule, and so on. This is because when an electron is removed, it leaves the overall charge on the atom or molecule to be positive and creates an attractive force between the already existing negative charge of the electron and the positive charge. As more electrons are removed from the atom or molecule, the positive charge increases and the attractive force between the positive and negative charge also increases. Therefore, the amount of energy required to release the electrons increases with the number of released electrons.
If I1 is the first ionisation energy, I2 is the second and I3 is third, then
I1 < I2 < I3
- For a particular element, the ionisation energy simply increases with the release of electrons one by one, but the trend of ionisation energy in the periodic table differs between the rows and columns. The ionisation energy increases in the left to the right direction for elements in a particular period, i.e. in the same rows. But the ionisation energy will decrease for elements of a particular group i.e. of the same column in the top to bottom direction.
The ionisation energy decreases in the downward direction for a particular group because, in that direction, the addition of an electron shell happens. This extra electron shell increases the distance of the outermost electron with the nucleus which makes the electron comparatively loosely packed, which results in less requirement of energy for the removal of the electron.
Exceptions in the trend of ionisation energy
In the chart of first ionisation energy that is shown above, 2 exceptions to the trend can be seen. The first one is that the first ionisation energy of boron is less than the first ionisation energy of beryllium and the second exception is oxygen, which has first ionisation energy less than that of nitrogen. Except for boron and oxygen, all the elements follow the general trend, i.e. first ionisation energy always increases in a left to right direction in a particular row or period.
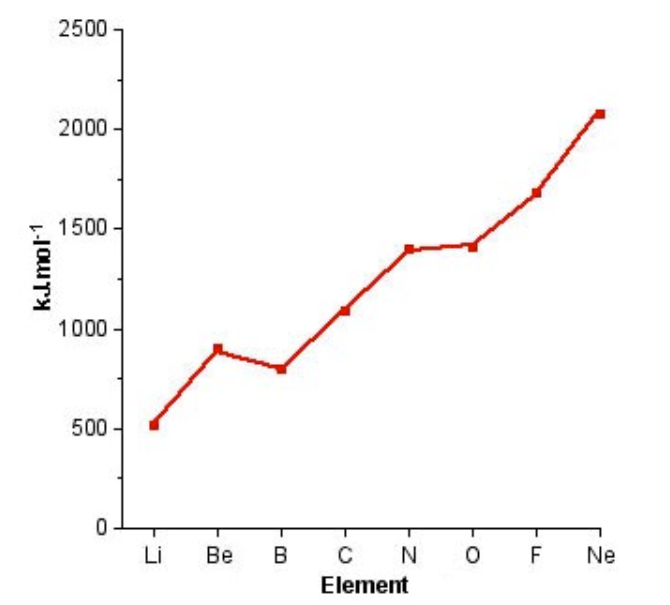
Ionisation energy reducing for Boron and Oxygen
This exception or discrepancy in the trend occurs due to the electron configuration and Hund’s rule. The electronic configuration of boron is 2s2 2p1, while the electronic configuration of beryllium is 2s2, which means that the first ionisation energy of beryllium comes from the electron in the 2s orbital and for boron, the first ionisation energy comes from the electron which is situated in the 2p orbital. So, due to the larger distance between the electron and nucleus in boron as compared to the distance of the electron-nucleus distance of beryllium, the first ionisation energy of boron is lesser than that of beryllium. The discrepancy between oxygen and nitrogen is due to Hund’s rule that defines the sequence in which the electrons will be filled. In both nitrogen and oxygen, the electron that has to be replaced is situated in the 2p orbital, but the electrons in the 2p orbital of nitrogen have the same spin while the oxygen has all electrons paired.
Note: In chemistry and physics, different measures are used for ionisation energy. In physics, ionisation energy is defined as the energy required to remove an electron from a single atom and it is measured as electronvolts. While in chemistry, ionisation energy is defined as the quantity of energy required by all the atoms present in a mole of a substance to release one electron each. In chemistry, it is also called molar ionization energy or enthalpy and it is expressed or measured as kilojoules per mole (kJ/mol) or kilocalories per mole(kcal/mol).
Factors affecting the ionisation energy
- Nuclear charge- With the increase in the nuclear charge, electrons get more tightly packed due to the positive charge of the ion and the negative charge on the electron.
- Type of orbital- Some atom that has a stable electronic configuration, will resist releasing an electron. Such atoms will have higher ionisation energy to overcome the stability of the electron configuration.
- Occupancy of the orbital- Like the stable orbital that is fully filled, the orbital that is half-filled will also have less tendency to release an electron. Atoms with such orbitals require higher ionisation energy.
- Effective nuclear charge- It is different from the general nuclear charge as this is the net positive charge on an electron in a multi-electron atom. The nuclear charge experienced is different for different electrons as the electron in the higher orbital is subjected to the shielding effect generated by the negatively charged electron, and doesn’t experience the full nuclear charge. Due to this “effective nuclear charge” on the particular electron is considered. With an increase in shielding, both effective nuclear charge and ionisation energy decrease.
- The number of electron shells- The number of electron shells shows the distance of the shell from the nucleus of the atom. If the shell with that number is far from the nucleus, then electrons will be loosely packed, and less ionisation energy will be required.
Considering the factors that affect the ionisation energy, let's predict which of the elements Neon, Oxygen, Magnesium, Sodium, and Fluorine has the highest ionisation energy and which element has the lowest ionisation energy.
The atomic number of the elements Neon, Oxygen, Magnesium, Sodium, and Fluorine are 10, 8, 12, 11, and 9 respectively. So, the configuration of these elements will be as shown below:
Neon- 1s2 2s2 2p6
Oxygen- 1s2 2s2 2p4
Magnesium- 1s2 2s2 2p6 3s2
Sodium- 1s2 2s2 2p6 3s1
Fluorine- 1s2 2s2 2p5
Among all the elements Magnesium and Sodium have the maximum number of shells, i.e. electron in these two elements is at the biggest distance from the nucleus. Now, between Magnesium and Sodium, only 1 electron is present in Sodium; therefore, it has the lowest ionisation energy.
The element that has the highest ionisation energy can be predicted by looking at the occupancy. Neon has its last orbital fully filled. Hence, it has the highest ionisation energy among all the elements.
Ionisation energy also explains the type of bond between the two elements. When the difference between the ionisation energy of 2 elements is high, they make an ionic bond. Sodium has ionisation energy of 496 KJ/mol, and the ionisation energy of Chlorine is 1251.1 KJ/mol. So, when they combine, they form an ionic bond and not a covalent bond. But elements like carbon and oxygen that have a very less difference in ionisation energy and are also close to each other on the periodic table are capable of forming a covalent bond.
Vertical and adiabatic ionisation energy in molecules
The vertical and adiabatic ionisation energy exists because the ionisation of any molecule changes the geometry of the molecules.
Adiabatic ionisation energy
The adiabatic ionisation energy of a molecule is the difference between the energy of the vibrational ground state of a neutral species and the positive ion. This can also be said as the minimum amount of energy required by an electron to leave a neutral molecule. The adiabatic ionisation energy is not affected by the specific equilibrium geometry of any species.
Vertical ionisation energy
With the possibility of change in molecular geometry due to ionisation, some additional transitions may occur between the vibrational ground state of neutral species and the vibrational ground state of the positive ion, i.e. ionisation comes with vibrational excitations. The intensity of these transitions can be understood by the Frank-Condon principle which says that the vibrationally excited state of the positive ion that has a similar geometry as the neutral molecule, has the most intense and better chances of transition. This is shown by a completely vertical line on the potential energy diagram, which it is called “vertical’ ionisation energy.
For diatomic molecules, neither adiabatic nor vertical ionisation energy is used to define the geometry. The length of a single bond in that diatomic molecule is used to define the geometry. Due to ionisation, i.e. removal of an electron from a bonding molecular orbit, the strength of the bond is reduced and the bond length is increased. Mostly the adiabatic ionisation energy is a more important physical quantity as it describes the difference in energy between the 2 potential energy surfaces. But due to some limitations to determine the adiabatic ionisation energy, vertical ionisation energy is used as it is easily identifiable and can be measured easily.
A similar trend between ionisation energy and electron affinity
While ionisation energy is for the removal of an electron, electron affinity is the energy required for the addition of one electron to a neutral atom and makes it a negative ion i.e. anion. Ionisation energy and electron affinity show a similar trend to each other in the periodic table. Both ionisation energy and electron affinity increase along the period and decrease down the group. The factor for their similar trend is also the same, i.e. shielding effect.
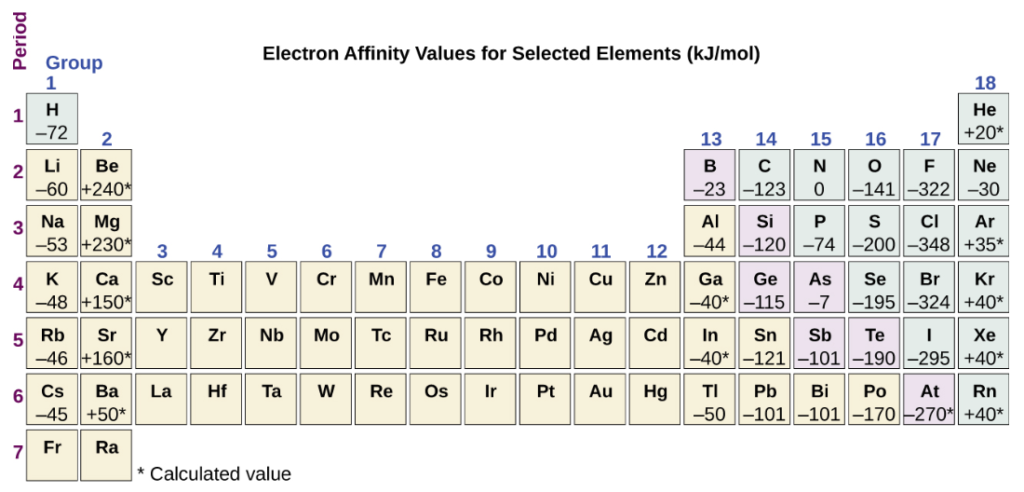
Electron affinity of some elements in the periodic table
Depending on the element, electron affinity can be endothermic or exothermic. The EA has been shown in the above picture for some elements, and we can see that there are many elements that have negative values of electron affinity. This negative sign shows that the energy is released when an atom accepts an electron. While there are elements with positive electron affinity also, means that those elements require energy to add an electron. Just like ionisation energy, electron affinity is also related to the forming ions with more charge and the second electron affinity is the energy associated with adding an electron to an anion with a charge of -1 to form an anion with a charge of -2.
We might think that the process of adding an electron becomes easier across a series of atoms due to the increase in effective nuclear charge. On moving left to the right across a period, electron affinity gets more negative. Also, just like the trend in ionisation energy of elements in the periodic table, there are exceptions in the trend of electron affinity too. For electron affinity, the discrepancies in the trend are found in group 2, group 15, and group 18. These exceptions are present due to the electronic structure. Group 18 consists of noble gases that have completely filled. Shells and due to this the incoming electron has to be added to a higher n level shell, which is more difficult to do. In group 2, s subshell is filled due to which the next electron addition has to happen in the p subshell with higher energy. In group 15, p subshell is half-filled due to which the next electron has to be paired with an existing p electron. So, the initial stability due to the electronic structure leads to these exceptions in the tend of electron affinity.
We might also think that the atom situated at the top of a particular group will have the most negative electron affinity, but in most groups, the second element has the most negative electron affinity. This is due to the shell with n = 2 that has small size and large repulsions between the electrons.
Example:
In group 17, chlorine is the element with the highest value of electron affinity of -348 KJ/mol in the periodic table and fluorine has an electron affinity of -322 KJ/mol. For fluorine when an electron is added to form fluorine anion, the addition of electron is done in n = 2 shell. This electron then gets attracted towards the nucleus, but due to the small size of the valence shell, it faces repulsion from the electrons that are already present. While chlorine also has the same electron configuration in the valence shell like fluorine, the electron addition in chlorine occurs in n = 3, which has a comparatively bigger size than that of n = 2 shell. So, electron-electron repulsion is less in chlorine due to which it excepts the electron more readily than fluorine.
Ionisation energy is useful in understanding the trend of the metallic property of metals too. Metallic properties, like conductivity and malleability, involve an electron that can be removed and ionisation energy is the energy required for it. The ionisation energy and metallic properties are inversely proportional to each other. So, metallic properties increase on going down a particular group, the direction in which ionisation energy decreases and decreases across a period.
Read more about electron configuration
Summary
- Ionisation energy is the energy required to release an electron. The atom compulsorily has to be in the gaseous state.
- First ionisation energy is less than the second ionisation energy and so on, due to the factor affecting the ionisation energy, nuclear charge, type of orbital, occupancy of the orbital, effective nuclear charge, and the number of electron shells.
- The value of ionisation energy increases along the period and decreases down the group with 2 exceptions boron and oxygen.
- When the difference between the ionisation energy of 2 elements is high, they make an ionic bond, and when the difference is less, they make a covalent bond.
- Ionisation changes the geometry of a molecule due to which adiabatic and vertical ionisation energy is used to define the geometry of such molecules.
- In diatomic molecules, neither of adiabatic and vertical ionisation energy can be used to define the geometry but rather the length of a single bond is used to define the geometry.
- Despite being the energy involved in exactly the opposite task, ionisation energy and electron affinity shows a similar trend in the periodic table.
- While in ionisation energy, exceptions in the trend are seen in group 13th and 16th, for electron affinity the exceptions are present in the group 2nd, 15th and 18th.
- Ionisation energy is inversely proportional to the metallic properties and can be used to predict the metallic properties of elements. More ionisation energy means less tendency to release electron, hence the element has less metallic property.
References
Wikipedia https://en.wikipedia.org/wiki/Ionization_energy
Openstax https://openstax.org/books/chemistry-2e/pages/6-5-periodic-variations-in-element-properties





