Table of Contents
Introduction to Hydrocarbons
Carbon and hydrogen are among the most crucial elements on earth as both forms the core of many biological processes. These natural processes over long periods produce compounds that exclusively contain carbon and hydrogen, and these compounds containing only carbon and hydrogen are known as hydrocarbons. The four general classes of hydrocarbons are alkanes, alkenes, alkynes and arenes.
The generalized term hydrocarbon gets mainly used to denote petroleum and natural gas in the oil and gas industry. IUPAC defines hydrocarbons according to the type of bonding between carbon and hydrogen. Accordingly, three classes of hydrocarbon are:
1. Saturated hydrocarbons are made entirely of single bonds, and these are also known as alkanes and are the basis of petroleum-based fuels. These can be either linear or branched, and these can be represented by a general formula CnH2n+2(1-r), where r represents the number of rings.
2. Unsaturated hydrocarbons are linear and branched compounds with a double or triple bond between the carbon atoms. A general formula CnH2n can represent these for noncyclic structures with only double bonds and CnH2n-2 for those having triple bonds. Double bonded unsaturated hydrocarbons get called alkenes, while those with triple bonds are called alkynes.
3. The third class of hydrocarbons are known as aromatic hydrocarbons or arenes. These contain pi-conjugated systems with rings having a variable number of carbon atoms.
Hydrocarbons containing more than one type of carbon-carbon bond get classified according to the most complex kind of bond. Hydrocarbons containing aromatic ring (s), triple bond (s), and double bond get classified as aromatic. In contrast, a compound with triple, double, and single carbon-carbon bonds gets classified as an alkyne. Non-aromatic hydrocarbons are often called aliphatic, which is further divided as paraffin (saturated hydrocarbons) and olefins (double-bonded hydrocarbons).
Uses of Hydrocarbons
Hydrocarbons are the major source of combustible fuels in the modern world.
1. Natural gas consists mostly of methane, while industrial fuels like jet fuel, gasoline, and naphtha mostly contain mid-sized chain alkanes and alkenes having 6 to 10 carbons.
2. Highly dense tar comprises long and complex aromatic and aliphatic compounds and is generally used for making roads.
3. Ethane and propane obtained from petroleum are converted to ethylene and propylene, serving as precursors to polyester polyethene, acrylates, and polystyrene.
4. Byproducts from the petroleum industry are used in various FMCG sectors like cosmetics, food, and detergents.
Alkanes and Alkenes in Common Usage
Methane is the simplest alkane and is the main constituent of domestic cooking gas. All alkanes are colourless. Alkanes with the lowest molecular weights are gases, those of intermediate molecular weight (for example, pentane) are liquids, and the heaviest (about 18 carbons) are waxy solids.
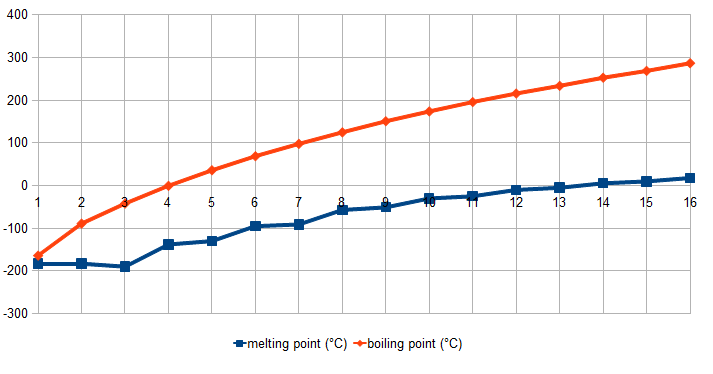

Melting points (blue) and boiling points (orange) of the first 16 n-alkanes at °C.
Methane is commonly used as cooking gas.

Plastics are made out of alkenes.
Since the electronegativity values of carbon and hydrogen are similar (2.5 and 2.1, respectively), the alkanes are typically non-polar. Hence, the only force that keeps their molecules together is the van der Waal's force, and therefore, the long-chain alkanes are dense, having high melting and boiling points. The insolubility of alkanes in water is also due to their non-polar nature.
Alkenes are prepared from alkanes via the cracking method. Thermal cracking takes place at high temperatures and high pressure. The carbon-carbon bond breaks, and an electron from the electron pair of covalent bonds goes to each carbon atom. As a result, shorter carbon chains, each with an unpaired electron, are produced. Because of lesser hydrogen atoms, one of the shorter chains must have a C=C and hence, is an alkene. Natural gas condensates are the most common raw materials for the cracking process. These are mostly ethane and propane in the US and Middle Eastern countries. In Asian and European countries, naphtha is used for cracking.

Another process similar to cracking is catalytic dehydrogenation, where an alkane loses hydrogens to form alkenes of the same order.
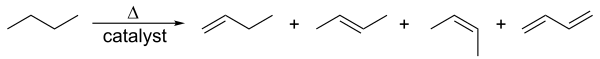
Isolation of Hydrocarbons from Fractional Distillation of Crude Oil
Crude oil is a mixture of various components that have different boiling points. Crude oil is refined, and its components are separated using fractional distillation.
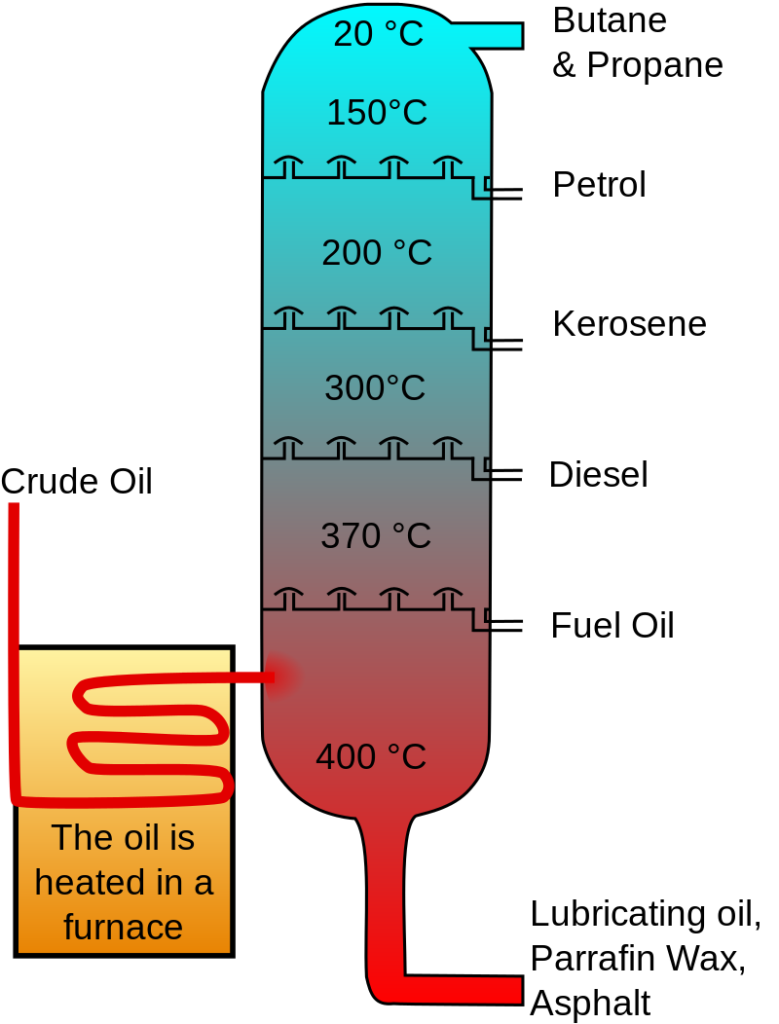
Fractional distillation of crude oil
The components with low boiling points, such as butane and propane, are vaporised easily at 20 degrees celsius, and on condensation, these are collected quite easily. Petrol and Kerosene are obtained above 150 and 200 degrees Celsius, respectively, while diesel and fuel oil are obtained above 300 and 370 degrees celsius. Lastly, above 400 degrees celsius, lubricating oils, paraffin wax, and asphalt are obtained.
Pollution caused by Hydrocarbons
Hydrocarbons are highly combustible, and hence their usage as fuels has caught up in the last 100 years at an ever-increasing speed. This has led to numerous environmental and health hazards.
Alkanes burn in the presence of excess oxygen and undergo complete combustion. The products of this combustion reaction are carbon dioxide and water. Carbon dioxide is the major source of the greenhouse effect and global warming. 2 Impurities in hydrocarbons lead to auxiliary pollutants like sulphur dioxide, causing acid rains.
3. In car engines, nitrogen and oxygen combine to form nitrogen oxides (NOx) because of spark and high pressure. NO is toxic and produces smog. NO2 is toxic and produces acid rain.
Read more about Infrared Spectroscopy
Frequently Asked Questions
What are hydrocarbons?
Hydrocarbons are a special class of compounds that are composed of carbon and hydrogen atoms. The simplest hydrocarbon is ethane (CH4). With more carbon atoms, the properties of each successive compound change.
How hydrocarbons are classified?
Hydrocarbons are classified into two classes according to the nature of the carbon chain; aliphatic and aromatic hydrocarbons. Aliphatics are those which have an open chain (e.g., methane) while aromatics have a closed chain/ringed structure (e.g., benzene)
What are the three basic classes of aliphatic hydrocarbons?
According to the no. of bonds between carbon atoms, aliphatic hydrocarbons are divided into three classes; alkanes, alkenes and alkynes having single bonds, double bonds and triple bonds between two or more carbon atoms, respectively.
How different hydrocarbons can be obtained from crude oil?
Different hydrocarbons can be obtained from crude oil by the process of fractional distillation. In this process, different hydrocarbons in crude oil are separated on the basis of their boiling points in a distillation tower.





