Table of Contents
Introduction to Chemical Equilibrium
Chemical equilibrium is a state in the course of a chemical reaction where the concentrations of both products and reactants reach the limit which prevents further deviation. Le-Chatelier’s principle is one of the pivotal ideas to understand the behaviour of a system in equilibrium. It states that “If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to partially reverse the change”. Another important idea which led to further development on this science, is the usage of Gibb’s free energy for quantifying the equilibrium. It is stated as “Equilibrium is attained when the Gibbs free energy of the system is at its minimum value (assuming the reaction is carried out at constant temperature and pressure)”. The equilibrium constant for a reaction finds its relation to the Gibb’s free energy as:
![]()
R is the universal gas constant and T is the temperature.
Le-Chatelier’s principle of equilibrium is used in the industrial applications as the reaction scheme involves parameters like temperature, pressure, concentration of reaction species a change in even single parameter results in the change of equilibrium leads to undesired product formation. The optimal parameter maintenance in the reaction scheme helps in achieving the desired product. Since the major product is highly desirable in industrial applications for profitability, unless the system yields the loss is effected due to undesired product. A mistake by operator of the process will result in costly loss to the process industry. The concentration of both reactant and product plays a vital role in equilibrium; for example, if the reactant concentration is higher it will lead to forward reaction and similarly a higher concentration of product leads to reverse reaction.
The parameter temperature, too has a role in determination of the reaction status. An endothermic reaction is experienced in the state of increased temperature and exothermic reaction scheme in decreased temperature state. Similarly, increased pressure causes decrease in volume of produce and vice versa.
In industrial chemical reactions, catalysts are most often used for increasing the yields of produce which has no effect on change of parameters but inert gas has an impact on reaction scheme and their implications are tabulated below for quick grasping.
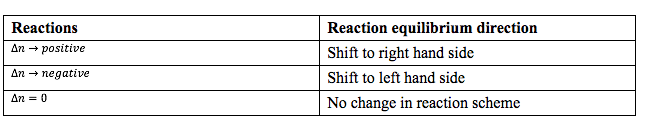
Key Points:
- Chemical equilibrium is a state in the course of a chemical reaction where the concentrations of both products and reactants reach the limit which prevents further deviation.
- Le-Chatelier’s principle - “If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to partially reverse the change”.
- Gibb’s free energy and equilibrium - “Equilibrium is attained when the Gibbs free energy of the system is at its minimum value (assuming the reaction is carried out at constant temperature and pressure)”.
- Catalysts have an impact on the time to reach equilibrium.
The main application of Chemical Equilibria in industrial process is to maximise the desired product concentration by minimising the leftover reactants.
Lime production from Limestone
![]()
In this reaction scheme the solid concentration is constant yielding equilibrium as
![]()
The reaction scheme is endothermic as it absorbs 178 kJ of heat in the form of energy for conversion to the desired product of CaO whose formation is influenced by high temperature making the reaction scheme feasible till it reaches the temperature of denoting the reaction scheme to be always favoured on the right hand side.
In addition, the gaseous carbon di-oxide is evolved from the reaction since there is no trace of gaseous reactant and hence the lowering of pressure favours the formation of more product. When compared to old kilns, modern rotary kiln favours high production rate of lime by continuous extraction of lime from the process.
Methanol Production
Methanol is used in fuel mixtures, urea-formaldehyde resin glue etc., and is a very important commercial chemical. It is produced by the reaction:
![]()
The reaction parameters for producing methanol are achieved by maintaining temperature at by using the catalysts of by maintaining reactor pressure at 5 MPa to 10 MPa. In the reaction scheme, 3 moles of synthesis gas is required for making one mole of methanol vapour which is further condensed to liquid state via condensing process. By Le-Chatelier’s principle, the high-pressure maintenance is the main parameter for the formation of the products and the inclusion of smaller amount of catalyst with moderate temperature maintenance favours the product formation of methanol. The kinetic expression based on pressure for the reaction scheme is,
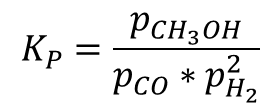
Frequently Asked Questions
What is chemical equilibrium?
Chemical equilibrium is a state achieved in reversible reactions when the concentration of the reaction mixture becomes constant due to equal rates of forward and reverse reactions.
What do you understand by Le-Chatelier's principle?
Le-Chatelier's principle explains the behaviour of a system in equilibrium in response to any disturbing change. It states, "If conditions are changed in a dynamic equilibrium to disturb it, equilibrium position changes to partially reverse the change."
How do chemical equilibrium and Le-Chatelier's principle help in the industrial preparation of compounds?
Le-Chatelier's principle helps to understand the nature of a chemical reaction and the position of chemical equilibrium. It helps to adjust the conditions of a chemical reaction, so that reaction yield is maximum.
Does catalyst affect the equilibrium state?
The catalyst reduces the time to attain equilibrium but does not affect the equilibrium position or the products' nature.
References:
P. W. Atkins, Physical Chemistry, 7th Ed.(2002) Oxford University Press, New York.
http://www.chemguide.co.uk/physical/equilibria/lechatelier.html
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Equilibria/Le_Chatelier's_Principle





