Table of Contents
The word “energetics” refers to heat or energy. Chemical energetics involves the transformation of heat during a chemical reaction.
Explanation of Chemical Energies
As we know that chemical reactions involve the breaking and making of bonds and in these processes, energy plays an important role. During bond breaking energy is required while in bond formation energy is released. Based on, we categorize chemical reactions as exothermic and endothermic chemical reactions.
The heat transformation during a chemical reaction is called enthalpy change. It is the key factor that determines whether a chemical reaction can happen or not.
Exothermic reaction
A reaction in which energy is released to the surrounding is called an exothermic reaction. The heat given off is indicated by a negative sign.
In this reaction, the energy required to break the is less compared to the energy given off during bond formation. In the form of a chemical equation, it can be written as,
C + O₂ → CO₂ + 393 Kj/mol
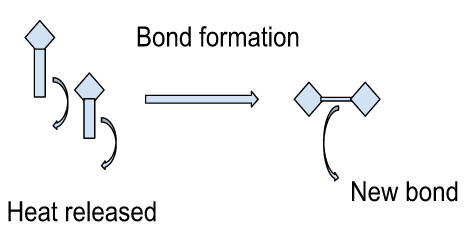
Examples
1: Neutralization reactions
It is the reaction in which acid and base react to form salt and water. Heat is also released in this reaction.
HNO2 + NaOH → NaNO2 + H2O + -∆H
2: Combustion of fuel
Fuels burn in the presence of oxygen and form carbon dioxide and water. Heat is also released.
CH4 + O2 → CO2 + H2O + -∆H
3: Oxidation of carbohydrates
Carbohydrates such as glucose react with oxygen and form carbon dioxide and water. Heat is also released.
C₆H₁₂O₆ + 6O₂ → CO2 + H2O + -∆H
Energy level diagram of exothermic reaction:
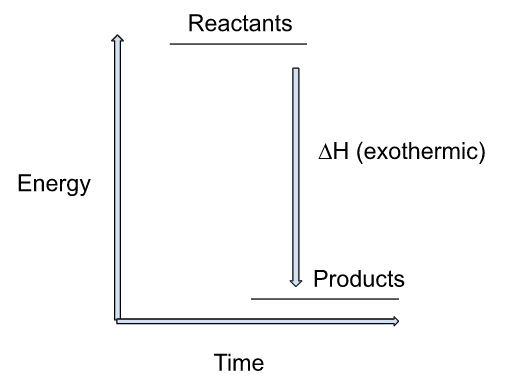
This energy level diagram shows that the energy of the reactant is high as compared to the product and heat is given off to the surroundings thus, ∆H will be negative.
Endothermic reaction:
The reaction in which heat is absorbed is called the endothermic reaction. The heat absorbed is indicated by a positive sign.
In this reaction, the energy required to break the bond is more compared to the energy used in the formation of the bond. In the form of a chemical equation, it can be written as,
C + H₂O + 131 kj/mol → CO + H₂
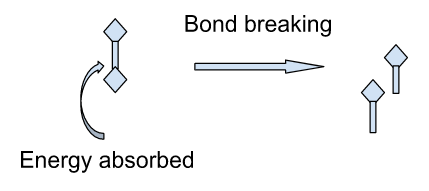
Examples:
1: Melting of ice
Ice absorbed heat to break the bond and thus water is converted from solid to liquid form.
H2O(s) + ∆H → H2O(l)
Electrolysis
It is the chemical decomposition of an electrolyte by passing the current.
NaCl + ∆H → Na+ + Cl-
Thermal decomposition of carbonates
Various carbonates absorb heat and undergo decomposition. Such as,
MgCO3 + ∆H → MgO + CO2
The energy of molecules that take part in a reaction is called internal enthalpy or enthalpy of the system while the energy that does not involve in a reaction is called enthalpy of surroundings.
Energy level diagram of endothermic reaction:
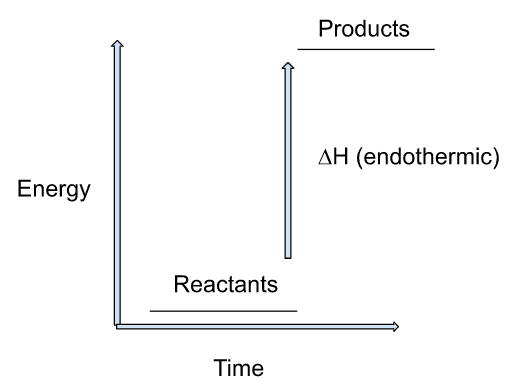
This energy level diagram shows that the energy of the product is high as compared to the reactant and heat is absorbed from the surroundings thus, ∆H will be positive.
Standard enthalpy changes:
The enthalpy change is simply the heat added or released during a chemical reaction at constant pressure while standard enthalpy change happens when the reaction takes place at the standard condition and everything that takes part in a reaction is at its standard condition.
The standard condition includes:
- Pressure at 100 KPa
- Temperature at 25°C
- All reactants must be present at their normal physical state
- In case of allotropes energetically stable one must be used
- Solutions at 1mol dm-3
The standard enthalpy change is symbolized as “𝚫H°”. This sign indicates that the reaction is taking place under standard conditions.
The standard enthalpy change of combustion of methane is -890.3 KJmol-1. It can be written as,
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) 𝚫H° = -890.3 KJmol-1
For every type of reaction standard enthalpy changes can be described with reference to their reaction name.
For example:
- Standard enthalpy change of reaction (general) (𝚫H°r)
- Standard enthalpy change of neutralization (𝚫H°n)
- Standard enthalpy change of formation (𝚫H°f)
- Standard enthalpy change of solution (𝚫H°sol)
- Standard enthalpy change of combustion (𝚫H°c)
- Standard enthalpy change of hydration (𝚫H°hyd)
- Standard enthalpy change of atomization (𝚫H°at)
Standard enthalpy changes of reaction (general) (𝚫H°r)
It is the standard enthalpy change that occurs when one mole of a substance is transformed during a chemical reaction at standard conditions.
Example:
2H2(g) + O2(g) → 2H2O(l) 𝚫H°r = -576 kJ mol-1
This equation indicates that the formation of water is an exothermic reaction and 576 kJ mol-1 is released when one mole of oxygen and two moles of hydrogen react with one another. The enthalpy change is determined by using Hess’s Law.
The general equation which is used as an expression of enthalpy of reaction is given below,
𝚫H°r = 𝚫H°(product) - 𝚫H°(reactant)
Read more about Chemical Energetics
Standard enthalpy changes of neutralization (𝚫H°n)
It is the standard enthalpy change that occurs when acid and base react during a chemical reaction and form one mole of water at standard conditions.
- In the case of neutralization between a strong acid and a strong base, the enthalpy change is constant because acid and base fully dissociate into ions. It could be considered the same reaction that occurs between OH- and H+ ions that completely react to form water. All other ions are spectator ions that are not involved in a reaction. Thus, the heat released during this reaction is the same.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) 𝚫H°n = -57.9 KJmol-1
In the case of each strong acid and strong base, the enthalpy change is almost similar.
- In the case of a weak acid, all acid is not ionized and the enthalpy change of neutralization also involves the enthalpy change of ionization and the enthalpy change of reaction between hydrogen and hydroxide ions. In weak bases such as ammonia again another enthalpy change and enthalpy of the reaction between hydrogen and hydroxide ions are involved because ammonia is present in the form of molecules. Thus, the weak acid and base enthalpy change of neutralization is less as compared to the enthalpy change of strong acid and base it means this is a less exothermic reaction.
NaOH(aq) + HCN(aq) → NaCN(aq) + H2O(l) 𝚫H°n = -11.7 KJmol-1
Standard enthalpy changes of formation (𝚫H°f)
It is the standard enthalpy change that occurs when one mole of a compound is formed during a chemical reaction from its elements at standard conditions. All the reactants and products must be present at their standard condition.
It could be exothermic or endothermic.
Example:
C(s) + O2(g) → CO2(g) 𝚫H°f = -393.41 KJmol-1
Carbon is present as graphite.
Standard enthalpy changes of solution (𝚫H°sol)
It is the standard enthalpy change that occurs when one mole of ionic substances gets dissolved into the solvent at standard conditions.
For example:
NaCl(s) + aqueous → NaCl(aq) 𝚫H°sol = +6.0 KJmol-1
It could be exothermic or endothermic.
Standard enthalpy changes of combustion (𝚫H°c)
It is the standard enthalpy change that occurs when one mole of a substance is completely combusted in the presence of oxygen at standard conditions.
It is always exothermic.
For example:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) 𝚫H° = -890.3 KJmol-1
Methane is burned in the presence of oxygen and 890.3 KJmol-1 energy is released. In the case of incomplete combustion carbon monoxide, water and soot are formed and the reaction is less exothermic.
Standard enthalpy changes of hydration (𝚫H°hyd)
It is the standard enthalpy change that occurs when one mole of ions in gaseous form is converted into an aqueous form at standard conditions.
It is always exothermic because a bond formation process occurs.
For example:
F-(g) + aq → F-(aq) 𝚫H°hyd = -506 KJmol-1
Ion-dipole forces are created because water is a polar molecule. The OH- ions are attracted to positive ions while H+ ions are attracted to negative ions. In this way, a bond is formed between water and ions.
Standard enthalpy change of atomization (𝚫H°at)
It is the standard enthalpy change that occurs when one mole of gaseous atoms is formed from the element at standard conditions.
For example:
Na(s) → Na(g) 𝚫H°at = +148 KJmol-1
Hess’ Law:
Hess’ Law states that the enthalpy change of chemical reaction does not depend upon the route followed by the reaction because initial and final conditions are the same.
The enthalpy change does not depend on which route is followed by the reaction it only depends upon the difference between the reactants and product enthalpies.
Consider a general reaction between A and C. The intermediate stage is B. According to this law, the enthalpy change will be the same whether the reaction proceeds in one step from A to C or a two-step from A to B and B to C.
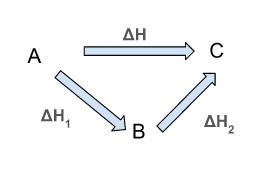
Possible Routes
By using Hess’ Law,
ΔH = ΔH1 + ΔH2
Consider a reaction between hydrogen and chlorine to form HCl.
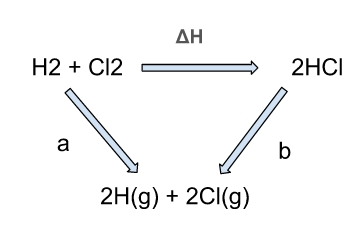
The arrows in the given diagram show the different routes that can be used during the reaction. The one route is shown by arrow “a” while the second is indicated through ΔH and “b”.
The mathematical expression could be written as,
a = ΔH + b or ΔH = a - b
Enthalpy change calculation:
Consider a reaction between aluminium oxide and magnesium.
Chemical equation:
2Al + 3Mg + 1.5O2 → Al2O3 + 3Mg
Al2O3 + Mg → 3MgO + 2Al
ΔH°f( MgO) = -601.7 KJmol-1
ΔH°f(Al2O3) = -1675.7 KJmol-1
Elements have 0 KJmol-1 ΔH°f
Equation used:
𝚫H°r = 𝚫H°f(product) - 𝚫H°f(reactant)
By putting values,
𝚫H°r = ( 3×-601.7) - ( -1675.7)
𝚫H°r = - 129.4 KJmol-1
Bond energies:
Bond energy is another important factor that helps to determine the enthalpy of a chemical reaction.
The energy required to break the bond is less compared to the energy released when new bonds are formed during the reaction, the reaction is exothermic.
If the energy required to break the bond is more as compared to the energy released during bond formation then the energy is absorbed from the surroundings and the reaction is called endothermic.
Bond energy is represented as “E”. The energy required to break the bond between the bromine molecule in a gaseous state is +193 KJmol-1.
Br2(g) → 2Br(g) E = +193 KJmol-1.
The energy value is always positive because energy is needed to break the bond.
Average bond energy:
The average bond energy is the energy required to break the bond into gaseous atoms.
The initial and final state of the substance must be in gaseous form. The term average bond energy is applied because the energy of every similar single bond is slightly different from one another. For example, the O-H bond in ethanol and methanol will have different energy due to different environments and C-H bonds in methane are also different from one another thus, we take the average bond energy to break them.
Average bond energies of some bonds:
H-H 435 KJmol-1
O-O 496 KJmol-1
C-H 410 KJmol-1
C-N 305 KJmol-1
Br- Br 193 KJmol-1
Cl-Cl 242 KJmol-1
Calculating Enthalpy changes by using average bond energies:
Consider the reaction between nitrogen and hydrogen.
Chemical equation:
1/2N2 + 1.5H2 → NH3
Average bond energies:
N-H -389 KJmol-1 (exothermic)
H-H +435 KJmol-1 (endothermic)
N≡N +945.36 KJmol-1 (endothermic)
𝚫H° = [0.5× E(N≡N) + 1.5× E(H-H) + 3× E(N-H) ]
𝚫H° = +472 + 652.5 -1167
𝚫H° = -42.5 KJmol-1
Experimentally measuring enthalpy changes:
Enthalpy change is measured by using a calorimeter and temperature change is measured with time as the reaction proceeds.
The equation used is given below:
q = mCpΔT
Q = Heat evolved or absorbed
m = Mass of substance
Cp = Specific heat capacity of a substance
ΔT = Change in temperature
Specific heat capacity:
It is the amount of heat required to raise the temperature of one mole of substance at standard temperature and pressure.
Consider that 0.56 g of propanol is completely combusted and heated in the water. The mass of water is 100 g and the temperature is increased from 25°C to 30°C. The amount of heat evolved would be determined by the above-given formula.
Mass of water = 100 g
Cp of water = 4.184 J/g.°C
ΔT = T2 - T1
ΔT = 30°C - 25°C
ΔT = 5°C
By putting values,
Q = 100 g × 4.184 J/g.°C × 5°C
Q = 2092 J
It is the energy change during the reaction.
Know we will calculate the enthalpy change.
The number of moles of propanol:
Number of moles = mass/ molar mass
Number of moles = 0.56 g / 60 g/mol
Number of moles = 0.009 mol
Enthalpy change of combustion:
𝚫H°c = Q / number of moles
𝚫H°c = 2092 J / 0.009 mol
𝚫H°c = 232.444 KJ/mol
Entropy:
Definition:
Entropy is a measure of the disorders of a system. The system with more disorders is more stable and energy spread is also greater.
It can also be described as the substances with more ways to share their energy have more entropy.
Explanation:
In matter states entropy increase in the following order,
Solid <liquid < gas < aqueous
It means the substances in aqueous states have higher entropy because particles are more disordered as states are changed and energy spread is also increased. These substances have more ways to arrange their atoms and energy thus their entropy increases.
Factors increasing the entropy:
Concentration:
As we increase the gaseous molecules entropy will increase because more molecules occupy more volume in space because of their disorder movement and energy spread out increases.
Temperature:
As we increase the temperature state change occurs which also leads to higher entropy. In case when the state is not changed by increasing the temperature entropy also goes to increase because by increasing the temperature kinetic energy of molecules also increases and they move faster and create more energy spread.
Formula:
It can be calculated by using the following formula.
ΔS = 𝝨Sᶿ(reactants) - 𝝨Sᶿ(product)
Thus, entropy is calculated by subtracting the standard entropies of the product from the reactant. It can be negative or positive. The negative value shows entropy is decreased while the positive values show that entropy of the reaction is increased.
Gibbs free energy
Definition:
It is the enthalpy of the system minus the product of temperature and entropy.
It is used to calculate the reversible work done by a thermodynamic system under constant temperature and pressure.
Formula:
ΔG = ΔH - TΔS
ΔG = change in Gibbs free energy
ΔH = change in enthalpy
T = temperature
ΔS = change in entropy
When the value of ΔG is less than or equal to zero reaction is spontaneous.
Feasible reaction
When entropy change is higher and the reaction is exothermic it will make the reaction feasible.
Conditions
ΔG will be negative
ΔS will be positive
ΔH will be negative
There is another possibility that when ΔG is negative, the reaction may not be feasible because in some cases activation energy is higher and the reaction does not proceed effectively.
Let's consider the following example:
Chemical equation
Al2O3(s) + 3C(s) → 2Al(s) + 3CO(g)
The change in entropy of a given reaction is +0.581 K-1KJmol-1. The change in enthalpy of reaction is +1336 KJmol-1. The reaction proceeded at 298 K. Gibbs free energy of the reaction will be,
Formula
ΔG = ΔH - TΔS
By putting values,
ΔG = +1336 - 298 (0.581)
ΔG = +1163 KJmol-1
It means the reaction is not feasible because ΔG is positive.
We can also calculate the temperature at which the reaction is feasible.
Let's consider the example:
Chemical equation
N2(g) + O2(g) → NO(g)
ΔH of a given reaction is 180 KJmol-1
ΔS of a given reaction is 0.025 K-1KJmol-1
As we know ΔG ≤ 0
Formula
ΔG = ΔH - TΔS
By putting values,
0 = 180 KJmol-1 - T (0.025 K-1KJmol-1)
T = 180 KJmol-1 / 0.025 K-1KJmol-1
T = 7200 K
References
- Goedhart, M. J., & Kaper, W. (2002). From chemical energetics to chemical thermodynamics. In Chemical education: Towards research-based practice (pp. 339-362). Springer, Dordrecht.
- Ott, J. B., & Boerio-Goates, J. (2000). Chemical Thermodynamics: Advanced Applications: Advanced Applications. Elsevier.
- https://igcsechemistryrevision.weebly.com/acids--energetics/-414-represent-exothermic-and-endothermic-reactions-on-a-simple-energy-level-diagram
- Brown, T. L., LeMay Jr, H. E., & Bursten, B. E. (2005). Chemical thermodynamics. Chemical Thermodynamics of Zirconium. NEA OECD, Elsevier, ISBN-13, 978-0.
- Stølen, S. (2004). Chemical thermodynamics of materials. Copyright.
- MISHIMA, S., MAETA, S., TATSUOKA, T., KOGA, N., & FURUKAWA, Y. (2009). Thermochemical Approaches to Neutralization Reaction between Weak Acid and Strong Base. Chem. Educ. J.(Web), 13(1), 13-4.
- Kodani, S., Fukuda, M., Tsuboi, Y., & Koga, N. (2019). Stepwise Approach to Hess’s Law Using Household Desiccants: A Laboratory Learning Program for High School Chemistry Courses. Journal of Chemical Education, 97(1), 166-171.
- Sutcliffe, R. (1983). Another method for solving problems based on Hess's law. Journal of Chemical Education, 60(4), 362.
- Smith, E. B. (2004). Basic chemical thermodynamics (Vol. 35). Imperial College Press.
- Astar Chemistry http://astarchemistry.com/cie/6-4bond-energy/
- Science Direct https://www.sciencedirect.com/topics/engineering/enthalpy-change
- Greaves, R. J., & Schlecht, K. D. (1992). Gibbs free energy: The criteria for spontaneity. Journal of Chemical Education, 69(5), 417.
- Chem Revise https://chemrevise.files.wordpress.com/2019/03/5-cie-chemical-energetics.pdf
- Rock, P. A. (2013). Chemical thermodynamics. University Science Books.





