Table of Contents
Summary
- The strength of an ionic bond is described by its lattice enthalpy.
- Lattice enthalpy explains many practical properties of ionic compounds including their solubility, hardness, and volatility.
- This lattice enthalpy is measured by Born-Haber Cycle.
- Born-Haber cycle is an application of Hess’s cycle which states that the enthalpy change in a chemical change is independent of the path by which the chemical change is taking place.
Atom can lose or acquire electrons to form ions and they do so to complete their valance shell electronic configuration and complete their octet in their outermost shell. Atoms that tend to lose electrons thus get a positive charge. The energy required to remove one electron from the valance shell of an atom in its gaseous state is called its first ionization energy. In a typical example of sodium, its electronic configuration is:
11Na23: 1s2, 2s2, 3p6, 3s1
Sodium loses one electron and in this process, it acquires a unit positive charge.

Similarly, atoms that tend to acquire electron gain unit negative charge and the energy released in acquiring an extra electron is called electron affinity. In the case of chlorine, its electronic configuration is
17Cl35: 1s2, 2s2, 2p6, 3s2, 3p5
Here chlorine prefers to acquire one electron to complete its outmost shell and in the process, it gains a unit negative charge forming a chloride ion (Cl-). The energy released is 355KJ/mol.

Lattice enthalpy
The resulting two ions (Na+ and Cl-) get into a columbic interaction (attraction between two opposite charges) and hence associate themselves in a chemical bond called an ionic bond. These ions arrange themselves in a structured pattern called lattice or ionic crystal. The strength of this ionic bond is given by lattice enthalpy. Higher the lattice enthalpy value, the stronger the ionic forces. It can be defined as the energy released when two gaseous ions are combined together to form one mole of a solid at 0K. In other words, it is energy acquired when one mole of solid is broken up to make its constituent gaseous ions. In both ways, the net amount of energy change would be the same, but with different signs. For example in the case of sodium chloride, we can write it down as:
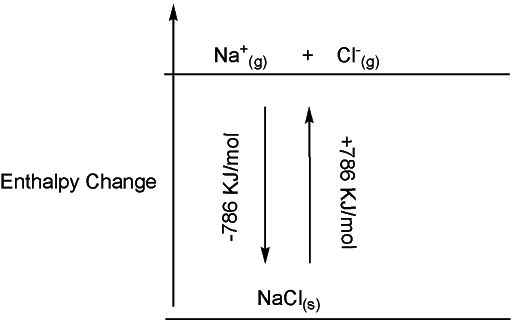
What does lattice enthalpy tell us?
Lattice enthalpy explains many practical properties of ionic compounds including solubility, hardness, and volatility. It describes the nature of bonding in a solid crystalline compound. We can define whether the bonding is purely ionic or not. However, it is impossible to measure the lattice enthalpy of solid as we cannot form solid from gaseous ions in practice. We can calculate lattice enthalpy by Born-Haber Cycle.
Read more about Lattice Enthalpy
Calculate lattice enthalpy using Born-Haber Cycle
Bonr-Haber cycle is an application of Hess’s cycle which states that the enthalpy change in a chemical change is independent of path by which the chemical change is taking place. Born & Haber both applied this law in determining lattice enthalpy. We can draw this diagrammatically as
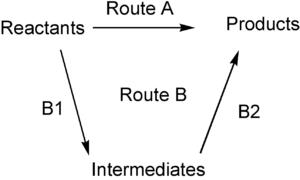
For Hess's cycle, the overall enthalpy change in these two routes will be the same. The enthalpy change for A would be the sum of all energies involved for steps B1 and B2.
HA = HB1 + HB2
So, if we know two values of enthalpy changes for the three separate reactions shown on this diagram (the three black arrows), we can easily calculate the third. This is what we do in Born Haber Cycle.
Let’s calculate lattice enthalpy for sodium chloride
The formation of sodium chloride from the elemental states at 25∘C and 1 atm can be described as

To calculate lattice enthalpy of NaCl(s) formation, we may have multiple steps as
Here we have, sodium(s) converted to its gaseous atoms and the enthalpy change is called heat of sublimation, sodium(gas) is then converted into sodium ion (Na+) and its first ionization energy is known to us. In other steps, we have chlorine atomized to give chlorine atom and subsequently to chloride ion (Cl-), and its heat of atomization and electron affinity are also available. The heat of formation of one mole of solid sodium chloride is also known. Then using this data, we can calculate the lattice energy using Hess’s cycle as

Let’s draw this in a cycle (Born Haber Cycle)
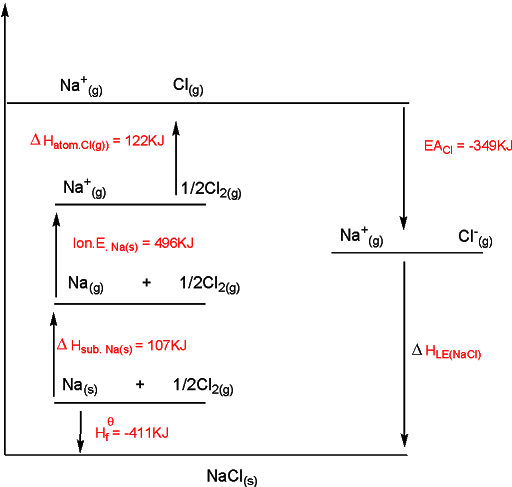
Now from Hess’s cycle, we know:
ΔHf0 = ΔHsub.Na + Ion.E Na + ΔHatom.Cl + Elec.ACl(g) + Lattice energy (LE)
Putting the energy values in the equation we get
-411 = 107 + 496 + 121+ (-349) + LE
-411 = 375 + LE
LE = -411 – 375
LE = -786 KJ/mol
So, the lattice energy for ionic sodium chloride is -786KJ/mol. The measured value is compared against the theoretical value obtained by physics calculation assuming ions as point charges. A close agreement endorses the ionic model of the lattice. For sodium chloride, lattice enthalpy by the Born-Haber cycle is in reasonable agreement with theoretical values. Any difference between measured values and theoretical value presents a degree of covalence.
Frequently Asked Questions
What is the Born-Haber cycle?
It is a cycle of enthalpy change of process leading to the formation of a crystalline lattice from the standard states of elements in such a way that the total enthalpy change of process remains zero.
Why is the Born-Haber cycle used?
The born-Haber cycle is an application of Hess's law to calculate the lattice energy of the ionic compounds.
What is lattice energy?
Lattice energy is the energy required to convert one mole of ionic compounds to gaseous ions.
What does lattice energy tell us?
Lattice energy tells us about many properties of ionic compounds, such as their bonding strength, stability, hardness and volatility etc.
Books for further study
- Shriver DF, Atkins PW, Langford CH: Inorganic Chemistry: 4th Ed. 1994. Oxform University press.
- Malik WM, Tuli GD, Madan RD. Selected topics in Inorganic Chemistry. 1998. S Chand & company.
- Catherine EH, Sharpe AG: Inorganic Chemistry. 2nd Ed. 2005. Pearsons ltd.
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





