Table of Contents
Key Information & Overview
- Definition
- Trends in the Periodic Table
- Common Reactions of different elements
- Entropy of Elements
Summary
- Elements are chemical substances which can not be broken down into simpler substances through chemical means.
- Each element contains one type of atom. An atom is the smallest part of an element that can take part in a chemical reaction.
- The patterns seen across period three are also seen across other periods. This recurrence of the same pattern is called periodicity.
- Down the group, the thermal stability of metal carbonates and metal nitrates of group II increases.
- The solubility of group II sulphates decreases down the group.
- The solubility of group II hydroxides increases down the group.
- The entropy of elements in a gaseous state is more than the elements which are in a liquid state. The entropy of elements in a liquid state is more than the elements which are in a solid state.
- Transition elements are d-block elements that make at least one ion with partly filled d-orbital.
- Group 17 elements are called halogens. Halogens have seven valence electrons. They exist as diatomic molecules.
Definition of an element
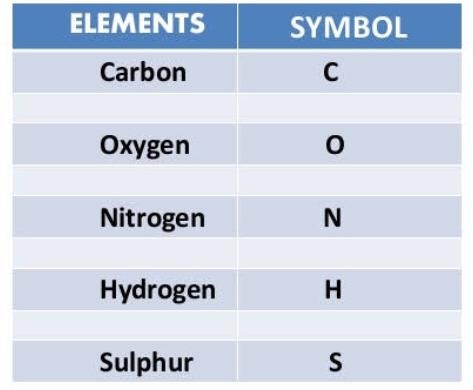
Elements are chemical substances which can not be broken down into simpler substances through chemical means. Different elements are represented by different chemical symbols. The table below shows some elements and their symbols.
Atom
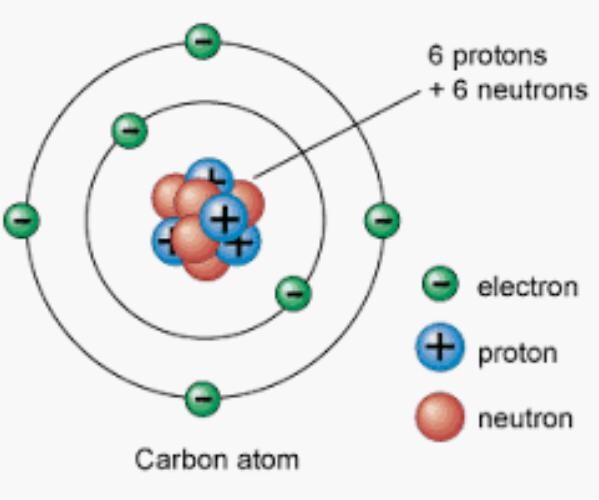
Each element contains one type of atom. An atom is the smallest part of an element that can take part in a chemical reaction. Atoms are made up of particles called nucleons (protons and neutrons). An atom consists of a nucleus surrounded by shells. Protons and neutrons are found in the nucleus and electrons are found in the shells surrounding the nucleus. The following diagram shows the structure of a carbon atom.
Periodicity (period 3 elements)
Elements are arranged in the periodic table in order of atomic number. Elements in the same group have the same number of valence electrons. Elements in the same period have the same number of shells. Group number tells us about the number of valence electrons of an element while the period number tells us about the number of shells occupied by those electrons. The patterns seen across period three are also seen across other periods. This recurrence of the same pattern is called periodicity.
The atomic radius of elements across period three decreases due to the increase in proton number and the same shielding.
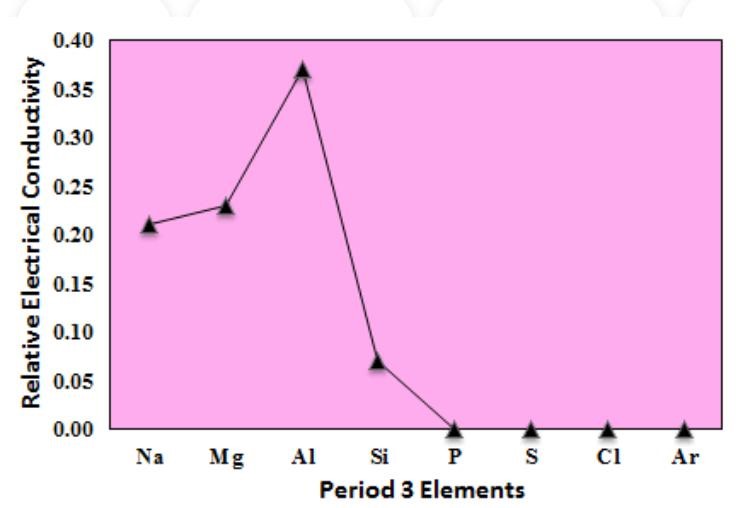
The electrical conductivity across period three increases from sodium to aluminium, as they have metallic bonding. Silicon, being a semiconductor, has a very low electrical conductivity. Phosphorus and sulphur are non-metals. They are good insulators and have no electrical conductivity.
In general, the ionisation energies of period three elements increase across the period due to the increase in the positive nuclear charge. Reactions of period 3 elements with oxygen:
- Sodium vigorously reacts with oxygen to form a white solid of sodium oxide. Sodium burns with a bright yellow flame.
- Magnesium reacts vigorously with oxygen to form magnesium oxide. Magnesium burns with a white flame.
2Mg(s) + O2(g) —> 2MgO(s)
- Powdered aluminium reacts with oxygen to form aluminium oxide. Aluminium burns with a white flame.
4Al(s) + 3O2(g) —> 2Al2O3(s)
- Silicon forms silicon dioxide by slowly reacting with oxygen.
Si(s) + O2(g) —> SiO2(s)
- Phosphorus reacts with oxygen to form phosphorus oxide. Phosphorus burns with a yellow or white flame.
4P(s) + 5O2(g) —> P4O10(s)
- Sulfur burns with a blue flame to form sulfur dioxide gas. Further oxidation of sulfur dioxide gives sulfur trioxide.
- Chlorine and Argon do not react with oxygen.
Group II elements
Down the group, the thermal stability of metal carbonates and metal nitrates of group II increases. As the size of the cation increases, its polarising power decreases. The upper cation (smaller) can polarise the carbonate anion or nitrate anion more. So they decompose at low temperatures and in less time.
The solubility of group II sulphates decreases down the group as the enthalpy change of solution is getting endothermic.
Enthalpy change = Enthalpy change — Enthalpy change
Of solution of hydration of lattice energy
Size of cation increases down the group. So enthalpy change of hydration and enthalpy change of lattice decrease. But the decrease in enthalpy change of hydration is more than the decrease in enthalpy change of lattice. So enthalpy change of solution is getting endothermic. Solubilities decrease.
The solubility of group II hydroxides increase down the group as enthalpy change of solution is getting exothermic.
Enthalpy change of solution = Enthalpy change of hydration — Enthalpy change of lattice energy
Size of cation increases down the group. So enthalpy change of hydration and enthalpy change of lattice decrease. But the decrease in enthalpy change of lattice is more than the decrease in enthalpy change of hydration. So enthalpy change of solution is getting exothermic. Solubilities increase.
Entropy
Entropy is the measure of the degree of disorder of particles, energy or electrons in a system at that particular temperature.
Entropy or elements in a gaseous state is more than the elements which are in a liquid state. The entropy of elements in a liquid state is more than the elements which are in a solid state.
Transition elements
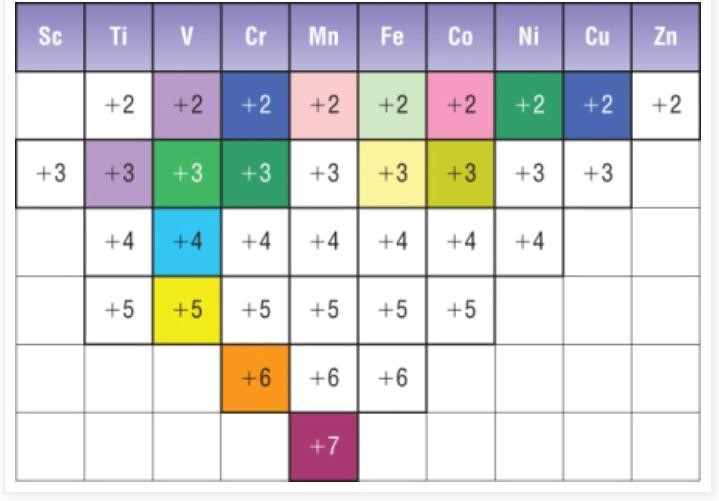
Transition elements are d-block elements that make at least one ion with partly filled d-orbital. Transition elements have variable oxidation states as 4s and 3d sub-shells have very close energy levels. So 4s, as well as 3d electrons are involved in bonding.
- +1 charge is only shown by copper
- All transition elements show +2 charge except scandium.
- All transition elements show +3 charge except zinc. Transition elements are d-block elements that make at least one ion with partly filled d-orbital. Scandium and zinc are not transition elements as they do not make ions with partly filled d-orbital.
Transition metal elements form coloured compounds as they have variable oxidation states.
Group 17 elements
Group 17 elements are called halogens. Halogens have seven valence electrons. They exist as diatomic molecules. Their melting and boiling points increase down the group as the temporary dipole-induced dipole forces increase down the group due to the increase in the number of electrons. The colours of the group 17 elements get darker down the group. Reactivity of halogens decreases down the group.





