Facts, Summary & Definition
- Axial and equatorial are types of bonds found in the chair conformation of cyclohexane
- The chair conformation is the most stable conformation of cyclohexane
- Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring
- The bond angles in this conformation are 110.9˚
What do axial and equatorial refer to?
Axial and equatorial are types of bonds found in the ‘chair’ conformation of cyclohexane. The chair conformation of cyclohexane has to lowest totally energy and is, therefore, the most stable.
Key Terms
To fully understand axial and equatorial bonds, there are some key terms that you must know.
Conformation: this is any shape that a molecule can take due to rotation around one or more of its bonds
Angle strain: this is strain due to bond angles that are not ideal and not of the lowest energy
Torsional strain: this is strain caused by repulsion. It can be reduced or completely eliminated by rotation around a single bond
Cyclohexane
Cyclohexane is a cycloalkane with a molecular formula of C6H12. This particular molecule has both the lowest angle and torsional strain of all the cycloalkanes.
The internal angles of a flat, regular hexagon are 120˚, as you can see in the image below. This causes problems when you consider that the preferred angle between bonds in a carbon chain is roughly 109.5˚.
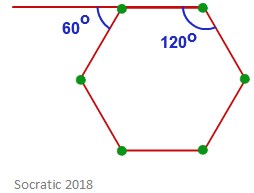
This means that a cyclohexane ring warps and takes a non-planar conformation in order to have a lower strain energy.
It can take up a number of non-planer conformations, the most common of which are the chair, half-chair, boat, and twist-boat. The conformation with the lowest strain energy is the chair conformation.
The Chair Conformation
As we have discussed earlier, the chair conformation of cyclohexane is the most stable because it has the lowest strain energy.
You can see the chair conformation of cyclohexane in the image to the right.
In this type of conformation, there are two positions: axial and equatorial.
Axial positions which perpendicular to the plane of the ring – these are highlighted in red on the diagram. The angles of these bonds are usually around 90˚.
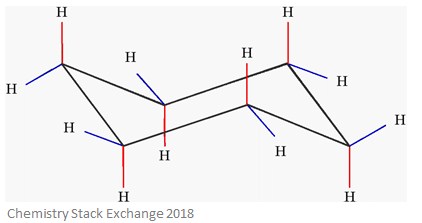
Equatorial positions are around the plane of the ring – these are highlighted in blue on the diagram. You can think of these bonds as radiating away from the ‘equator’ of the ring – this will help you remember their name.
It is important to notice that axial positions next to each other point in opposite directions. The same is also true for equatorial positions. This is shown more clearly on the diagram below.
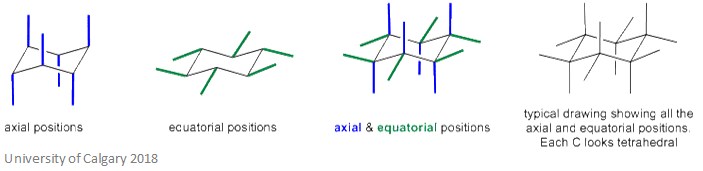
This conformation means that the bond angles are 110.9˚. Whilst this is not perfect, it is much closer to the ideal bond angle of 109.5˚. Also, all of the C-H bonds are fully staggered which eliminates the torsional strain in the molecule. These two features mean this conformation is incredibly stable.
You will need to know how to draw this conformation. The guide below shows you how to do this.
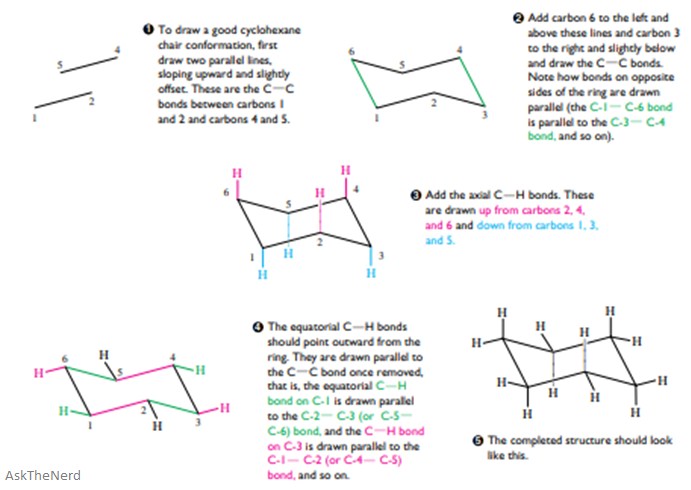
Boat Conformation
The boat conformation of cyclohexane, shown below, is a lot less stable than the chair conformation.
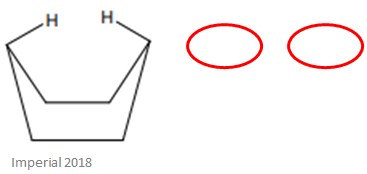
Whilst this conformation has nearly no angle strain, it does have a lot of torsional strain – this is because of the eclipsed bonds at the four carbon atoms forming the ‘side’ of the boat. The reason for the lack of angle strain is because all of the C-C bond angles, much like the chair conformation, are 109.5˚.
When hydrogen atoms are eclipsed, it means they are in the closest proximity to each other that they can be in – it is the opposite of the staggered conformation.
In this conformation, the two bonds highlighted in red are called ‘flagpole’ bonds – this makes the hydrogen atoms ‘flagpole’ hydrogens. The close proximity of these two hydrogens causes a lot of steric strain.
The boat conformation is an intermediate between the chair conformation and the twist-boat conformation.
Twist-Boat Conformation
The strain in the boat-conformation can be minimised somewhat by twisting it. Whilst it relieves some of the torsional strain and the steric strain, it does introduce more angle strain. It is more stable than the boat conformation, but less stable than the chair conformation. The twist-boat conformation can be seen in the image below.

Through the rotation of bonds in this structure, a huge proportion of the Van der Waals strain is reduced.
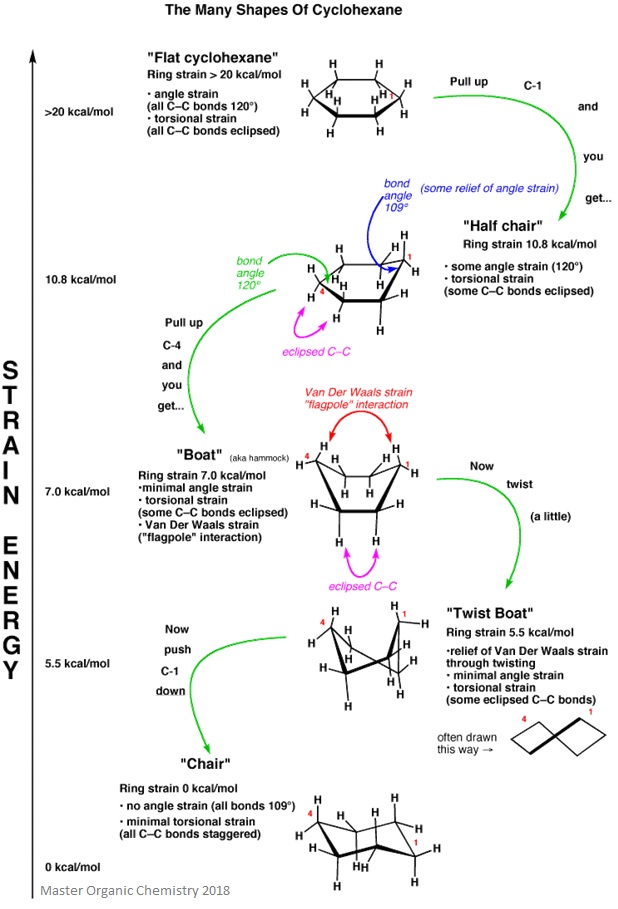
Strain Energy
The image below gives a fantastic overview of the strain energy in each conformation of cyclohexane.
Further Reading
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch03/ch3-06.html
http://www.chem.ucla.edu/~harding/ec_tutorials/tutorial15.pdf
http://www.chemtube3d.com/STcyclohexane%20ring%20flip.html
https://study.com/academy/lesson/cyclohexane-structure-formula-conformations.html
https://www.masterorganicchemistry.com/2014/04/18/ring-strain-in-cyclopentane-and-cyclohexane/





