Facts, Summary & Definition
- Allosteric enzymes are enzymes which have an additional site for an effector to bind to, as well as the active site
- Efforts regulate the activity of the enzyme – they can either activate or inhibit
- Allosteric enzymes are larger and more complex than normal enzymes
- They are regulated through homotropic regulation or heterotropic regulation
What are allosteric enzymes?
You will remember that enzymes are classed as biological catalysts. That is, they help to accelerate the rate of a reaction, but remain unchanged during the entire process.
Allosteric enzymes are enzymes which have an additional site, as well as the active site – it comes from the Greek ‘allo’, which means ‘other’. These are called allosteric sites, and enzymes can have more than one. They are unique in that they have the ability to respond to multiple different conditions in their immediate environment. Also, when allosteric enzymes are shown on a graph as velocity against substrate concentration, they show a sigmoid curve rather than the usual hyperparabolic curve.
The image below shows a generic allosteric enzyme.
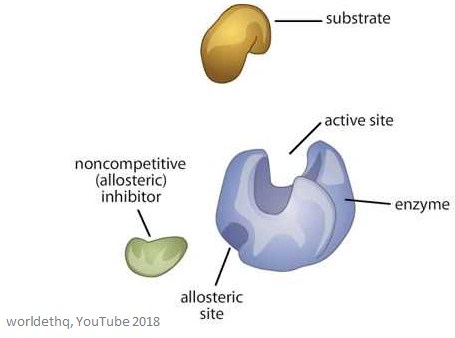
Properties of allosteric enzymes
Allosteric sites are binding sites on the enzyme – they are different from the active site and the substrate binding site.
The molecule that binds to the allosteric site is called an effector (it can also be called a modulator), and it regulates the activity of the enzyme it binds to.
The activity of the enzyme is increased when a positive allosteric effector binds to the allosteric site. This means that the activity of the enzyme is decreased when a negative allosteric effector binds to the allosteric site – they inhibit the enzyme.
Allosteric enzymes are larger and more complex than non-allosteric enzymes and often have many sub-units. Enzymes with more than one effector have different and specific binding sites for each one. In most allosteric enzymes, the substrate binding site and the effector binding site are on different subunits.
The substrate binding site is on the catalytic subunit – often referred to as the C subunit. The effector binding site is on the regulatory subunit – often referred to as the R subunit.
When an effector molecule at one binding site causes a conformational change it that subunit, a conformational change is then caused in the other subunits in the protein – this means that a huge portion of the binding energy of the effector is used to change the conformation of the whole protein complex.
This interaction between all of the subunits can be expressed using the Hill coefficient – this is also called a cooperativity coefficient. When n=1, there will be no interaction between the subunits in the enzyme. The larger the Hill coefficient (cooperativity coefficient), the stronger the interactions between all of the subunits in the enzyme.
Allosteric enzymes can also ‘switch’ between their active form and their inactive form. This allows for sophisticated response patterns in activity, which can play a huge role in biological function. Once the effector dissociates from the binding site, the enzyme is then able to revert back to its inactive (or less active) form. They can control the rates of highly important reactions, such as ATP production.
When an effector binds to an enzyme, it is called cooperative binding.
Homotropic Regulation
A homotropic allosteric effector is a substrate for the enzyme, as well as a regulatory molecule – the prefix ‘homo’ refers to them being the same. They are usually activators of the enzyme. The below image shows a homotropic allosteric effector.
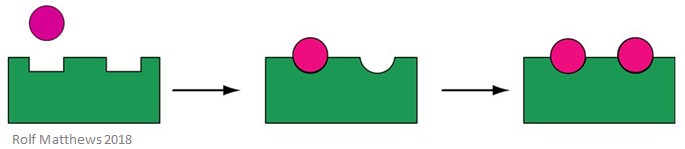
A good example of a homotropic allosteric effector is oxygen (O2) – it acts as an effector of haemoglobin in the human body.
Heterotropic Regulation
A heterotropic allosteric effector is a regulatory molecule which is not also the substrate for the enzyme. It can either activate or inhibit the enzyme it binds to. The below image shows a heterotropic allosteric effector.
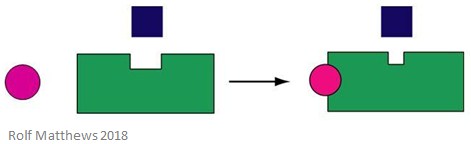
A good example of a heterotropic allosteric effector is carbon dioxide (CO2) – it also acts as an effector of haemoglobin but is not the enzyme’s substrate.
Essential Activators
Essential activators are allosteric activators that, without which, the enzyme activity would be so low it would be negligible. For example, N-acetylglutamate is an essential activator for carbamoyl phosphate synthetase I. They are the exact opposite of enzyme inhibitors.
Further Reading
https://www.khanacademy.org/science/biology/energy-and-enzymes/enzyme-regulation/a/enzyme-regulation





