Table of Contents
A chemical bond is a type of attraction between atoms, molecules, or ions that is the basis for the formation of all chemical compounds.
There are 4 major types of chemical bonds which are represented below:

Types of Chemical Bonds
Ionic Bond
Compounds that are composed of ions are referred to as ionic compounds, and the bond between the constituent ions is called an ionic bond.
Ion is an electrically charged particle that results when an atom loses or gains an electron.
There are two types of ions:
- Cations – positively charged ions
- Anions – negatively charged ions
Ionic bond results from electrostatic forces between oppositely charged anions (positively charged cations and negatively charged anions).
Let’s consider the mechanism for ionic bonding in the case of an ionic compound NaCl.
First, we should write the electron configuration for both atoms in the molecule.
Na + Cl ???? Na+Cl-
Na ???? 1S22S22P63S1 Na+ ???? 1S22S22P6
Cl ???? 1S22S22P63S23P5 Cl- ???? 1S22S22P63S23P6
As you can see, to form an ionic compound NaCl, Na loses its 1 electron from the outer subshell (highlighted in red) while Cl gains 1 electron, so its outer subshell is complete (highlighted in blue).
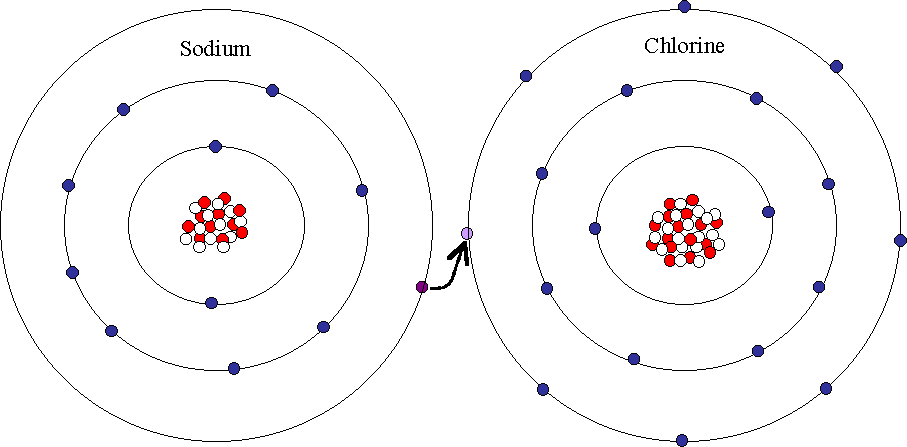
This electron transfer is the reason for the electrostatic force, which holds the positively and negatively charged ions together in an ionic molecule.
We can conclude that an ionic bond involves a transfer of an electron from one atom to another. As a result, one ion in the molecule carries a positive charge, while the other one takes a negative charge. Since oppositely charged particles attract, these two atoms with opposite charges attract each other to form an ionic bond and ionic compound.
Two features characterize an ionic bond:
- Bond length – the distance between the centers of the two ions
- increases as we go down the group
NaF < NaCl < NaBr
- decreases as we go along the period
NaCl > MgCl2 > AlCl3 - Bond energy – the energy required to break down all bonds in 1mol of substance
- decreases as we go down the group and along the period
Ionic compounds form ionic crystals lattices, which represent the arrangement of ions in regular, geometric structure. You can see a NaCl crystal below:
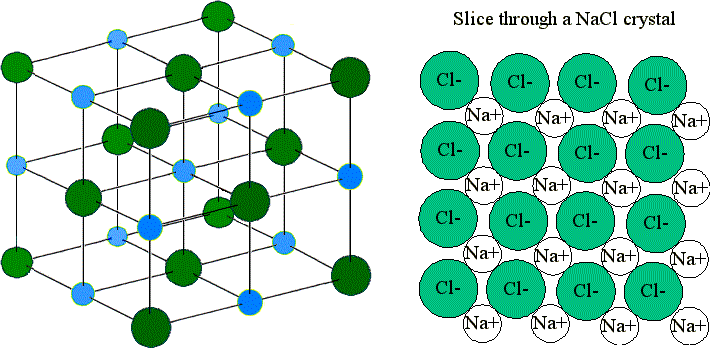
Since the attractive forces between the ions have the same magnitude in all directions, the forces are stated to be isotropic. Therefore, the ion in the molecule is equally attracted to every nearby ion with an opposite charge. This is the reason for the tightly bound, 3D lattice structure of ionic compounds.
Read more about Bonds and Structures
Covalent Bond
A covalent bond involves the equal sharing of electrons between two atoms in a molecule. Covalent bonds are formed in case if tendencies to attract electrons are similar for both atoms in a molecule.
A covalent bond is formed because all atoms desire to have the outer shell completed (have 8 valence electrons); therefore, they become stable. For that reason, atoms share electrons through chemical bonding to reach a stable configuration.
There are 2 major types of the covalent bond, which are the following:
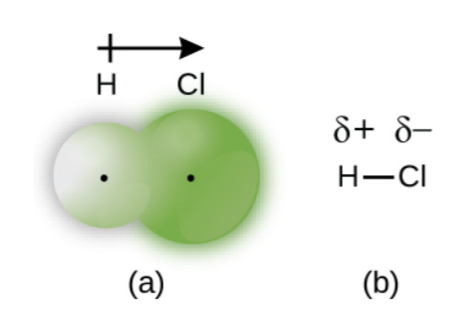
- Polar covalent bond – is formed between the two nonmetals with different electronegativities (tendency of an atom to attract electrons/electron dencity towards itself).
- Water is a perfect example of a polar covalent bond
- The picture below might help you to better visualize the concept
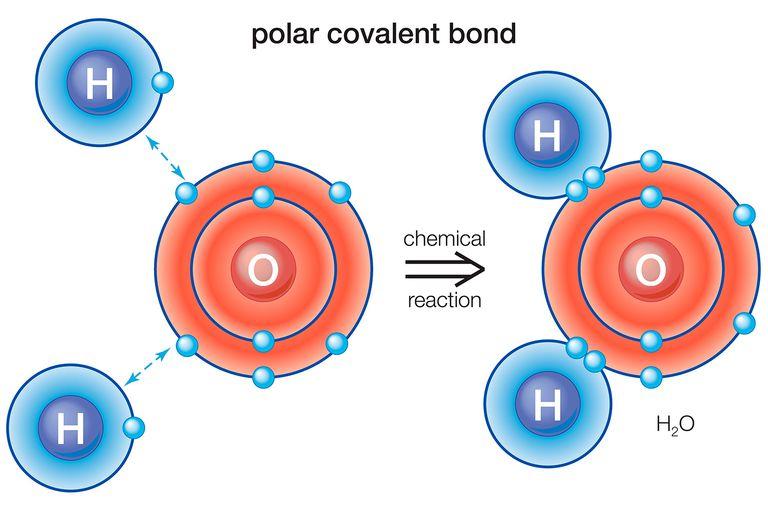
- Since the electrons through which the bond is formed are distributed unequally (due to the difference in electronegativities), the molecule has an electrical dipole moment; meaning than one end of the molecule is slightly positive (denoted by δ+ and the other one is somewhat negative (denoted by δ-).
- A nonpolar covalent bond also referred to as pure covalent bond – formed between two identical (same electronegativity values) nonmetal atoms through equal electron sharing. In this case, the probability of being near each nucleus is equal for the shared electrons.
- H2, O2, Cl2, Br2, F2 are several examples.
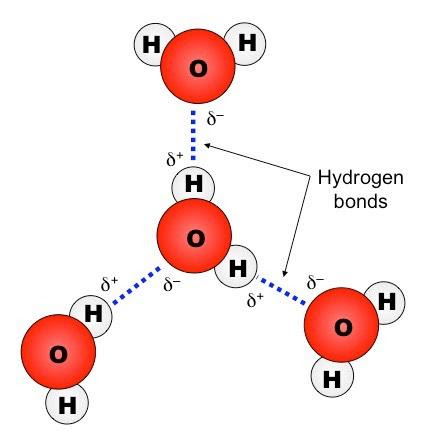
Hydrogen Bond
A hydrogen bond is formed between molecules composed of electronegative elements. For instance, H2O molecules are associated with each other through hydrogen bonding. The liquid form of H2O is described precisely by the presence of hydrogen bonds between H2O molecules.
There are 2 types of hydrogen bonds:
- Intramolecular hydrogen bonds – present within molecules (proteins)
- Intermolecular hydrogen bonds – present between molecules (H2O, HF, NH3, alcohols)
Hydrogen bonds between the molecules are represented as dots.
Metallic Bond
A metallic bond is formed in the case of metals that form metallic lattices in which positively charged metal ions are surrounded by electrons. This lattice structure and the fact that metal ions are attracted to the delocalized electrons grants metals high melting and boiling points.
Metals are the best electricity conductors due to the motion of the electrons.
The picture below might help you to better visualize the general structure of metallic lattices:

Summary
| Ionic Bond | Forms between ions by transferring electrons from one atom to another. |
| Covalent Bond | Forms between nonmetals by sharing electrons between each other.Polar covalent bond - Forms between 2 nonmetals with different electronegativities (or two different atoms); Nonpolar covalent = pure covalent bond - Forms between 2 identical atoms (with the same electronegativities) |
| Hydrogen Bond | Forms between molecules composed of atoms with high electronegativities. Intramolecular – present in protein; intermolecular – present between H2O, HF, NH3 molecules |
| Metallic Bond | Present in metals, granting rigidity and the ability to conduct electricity. |
A chemical bond can be characterized by the strength which is the measure of the energy required to break the bond between two atoms. If the bond energy (also referred to as bond enthalpy) is higher, it means that the bond is stronger. Similarly, a low bond energy value depicts that the bond is weak; therefore, it is quite easy to break it.
Generally, single bonds are longer in length and weaker in strength than double and triple bonds.
For example, C-C single bond has a length of 1.54Å and bond energy of 345kJ/mol. On the other hand, C=C double bond has a bond length of 1.34Å and bond energy of 611kJ/mol. As you can see, as the bond length decreases, the bond energy increases; therefore, the strength of a bond also increases.
Similarly, in the case of a C-N single bond, the bond length is 1.43Å and the bond energy is 290kJ/mol. C=N double bond has a bond length of 1.38Å and a bond energy of 615kJ/mol.
As a result, we can prove that the strength of a bond increases with decreasing the bond length. This happens because the shorter bond length means that the two atoms are close to each other; thus, it is harder to separate them.
In terms of strength, a hydrogen bond is the weakest bond. In the case of covalent and ionic bonds, covalent is considered to be stronger since it involves the sharing of 2 or more valence electrons. Since an ionic bond involves a transfer of an electron, the interactions between the two bonding atoms are considered to be weaker.
Frequently Asked Questions
What are the major types of chemical bonds?
Four major types of chemical bonds include ionic bonds, covalent bonds, metallic bonds and hydrogen bonds.
What is an ionic bond?
An ionic bond is formed by the complete transfer of electrons from one atom (donor) to the other atom (recipient).
How is a covalent bond formed?
A covalent bond is formed when two atoms mutually share electrons to get stability. For example bond between two chlorine atoms in Cl2 is covalent and formed by mutual electron sharing.
Which bond is strongest?
The covalent bond formed by mutual electron sharing is considered the strongest in chemistry.
References:
Blei, I., Odian, G. (2015). “Introduction to General Chemistry.”
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from:
http://cnx.org/content/col11760/latest/Zumdahl, S.S. (2019). “Water.” Retrieved from: https://www.britannica.com/science/water





