Table of Contents
Summary
Thermochemistry is a branch of Thermodynamics, a scientific field that comprises the relationships between heat, work, and other forms of energy resulting from different chemical and physical processes. Thermochemistry itself is defined as energy changes when a chemical reaction takes place.
Energy is the capacity to supply heat or do work. Thermochemistry is concerned with the energy (usually in terms of heat) absorbed or released during chemical or physical changes.
There are 3 general types of systems which are the following:
- Open System – involves the exchange of mass and energy/heat with its surroundings;
- Closed System – involves the transfer of energy/hear but not mass;
- Isolated System – does not allow the transfer of either energy/heat or mass.
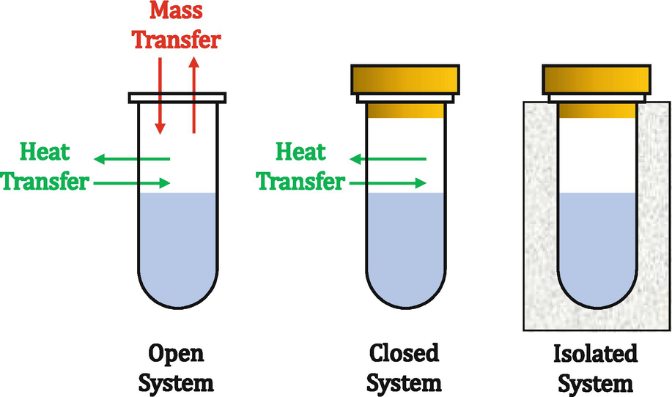
Thermochemistry for A-Level Chemistry focuses on the closed system, so we assume that there is no mass loss during the reaction.
There are 2 types of reactions:
- Exothermic – heat is released during the reaction;
- Endothermic – heat is absorbed during the reaction.
The same concept might be stated in a little bit different way:
- Energy is ether lost or gained in a chemical or physical process;
- Although, the energy is not destroyed nor created in either of the cases; the total energy of the universe remains constant at all times;
- Energy just transfers from one place to another;
- If the energy is lost during the reaction, it is an exothermic reaction – meaning that the energy gives the energy to its surroundings. As a result, the temperature is increased; therefore, the heat is released in the reaction;
- If the energy is gained during the reaction, it is an endothermic reaction – meaning that the reaction gets the energy from its surroundings. In such cases, we have to provide energy (usually in a form of heat)which can be absorbed during the reaction for the chemical process to occur.
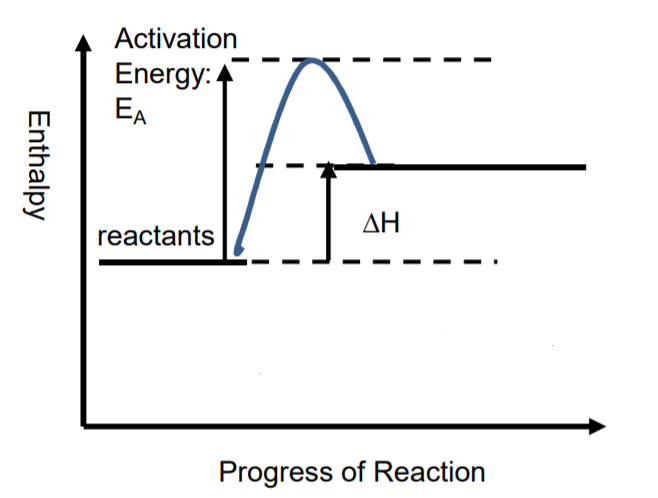
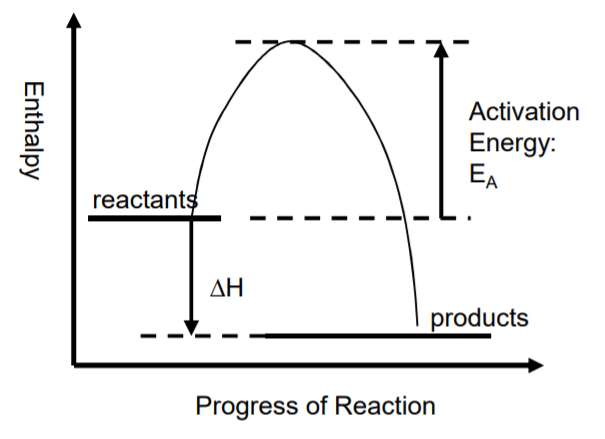
Let’s consider the energy level diagrams (also referred to as the reaction profiles) for exothermic and endothermic reactions:
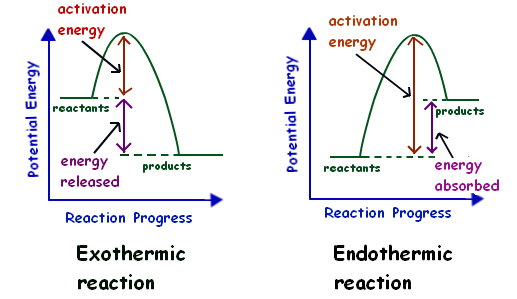
As you can see, in the case of an exothermic reaction, products at the beginning of a reaction have high potential energy and the energy is decreased as the reaction progresses. The difference between the energies of reactants and products is the energy that is released during the reaction. But still, there is the minimum energy required for a reaction to progress. This minimum energy is referred to as the activation energy.
An endothermic reaction is the opposite of an exothermic reaction that we have just discussed. In the case of an endothermic reaction, reactants have very low potential energy; therefore, they need the input of energy (activation energy) for the reaction to progress. As a result, the products that are obtained in a reaction have high potential energy. The difference between the energies of reactants and products is the energy that is absorbed during the reaction.
Read more about structures and reactions of organic compounds
Thermal Energy, Temperature, and Heat
Thermal Energy is a form of kinetic energy associated with the random motion of atoms and molecules. Increasing the amount of thermal energy increases the temperature of the system and vice versa.
The transfer of thermal energy between two borders with different temperatures is referred to as Heat (q). Transfer of heat always occurs from a subject with higher kinetic energy to a subject with lower kinetic energy.
Calorimetry
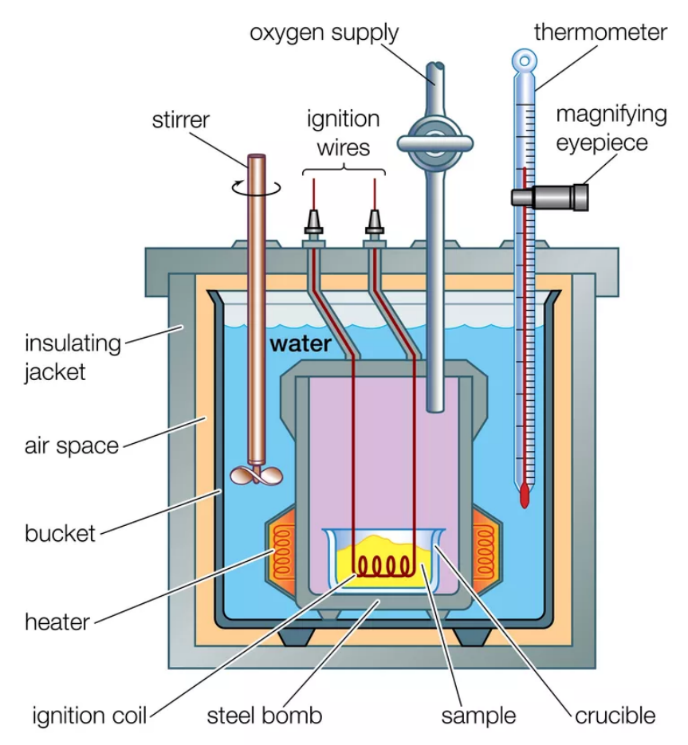
A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a closed container with a thermometer to measure the initial and final water temperature.
Energy is measured in units of Calories (cal). 1cal is the amount of energy required to increase the temperature of 1g of water by 1oC or 1K.
The SI unit of heat, work, and energy is the Joule (J). 1J is the amount of energy used to move an object for 1meter with a force of 1newton.
1cal = 4.184J
Heat Capacity
The heat capacity of a matter is a quantity of heat (q) absorbed or released due to the temperature change of 1oC or 1K.
C = q∆T , J/oC
Therefore, the value of heat capacity might also be dependent on the amount of the substance. To define this quantity, we use the term “Specific Heat Capacity” – the amount of heat required to raise the temperature of 1g of a substance by 1oC or 1K.
C = qm∆T , J/goC
From the equation above, we can derive the following equation for Heat (q):
q = C∙m∙∆T
Where:
q – the amount of heat, or heat change measured in Joules (J)
C – specific heat capacity (given constant for a particular substance), measured in Joules per Kelvin per gram (J∙K-1∙g-1)
m – mass of a substance that is heated, measured in grams (g)
- useful conversion: every 1cm3 of water is equal to 1g of water
∆T – temperature change = Tfinal – Tinitial; measured in Kelvin temperature
Kelvin temperature = oC + 273
Let’s consider the following sample problem:
Sample Problem:

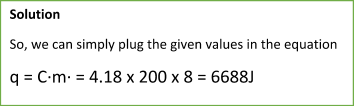
In such a problem, you might also be asked to provide the value of calculated heat in kJ/mol. This would be the enthalpy change of a reaction. In such a case, you will simply convert the mass of ethanol to moles. To do so, divide the mass of ethanol by the molar mass of ethanol.
For this sample problem, we would get the following:
n (ethanol) = mM= 0.46g46g/mol = 0.01mol
Now, we convert 6688J to kJ:
1kJ = 1000J → 6688J = 6.688kJ
And finally, we find the value of heat in kJ/mol by dividing 6.688kJ by 0.01mol of ethanol and we get the following:
668.8kJ/mol
Since the reaction is exothermic, we should have a minus in front of 668.8kJ/mol. So the final answer would be the following:
∆H = -668.8kJ/mol
NOTE:
Since the problem gives the temperature change, there is no need to convert the value to Kelvin. The reason is that if we are given 2 temperature values (initial and final) in Celsius and we convert both values to Kelvin, the difference between the two values will be the same.
In case if we are not looking for the temperature change, the temperature value provided in Celsius must be converted into Kelvin to get the proper result.
Enthalpy
Enthalpy (H) is a quantity used to define the total amount of heat of a system.
H = U + PV
Where:
H – enthalpy
U – internal energy of a system (sum of all types of energies present in a substance)
P – pressure
V – volume
Considering the fact that Enthalpy (H) is derived from 3 state functions (U, P, and V), Enthalpy is also a state function. Therefore, only enthalpy changes for a chemical or physical process can be measured.
So, the equation for H is used in the following form:
∆H = ∆U + P∆V
Where:
∆U = q + w = q - P∆V and P∆V = -w
+q → heat flows in the system;
-q → heat flows out the system;
+w → work is done on the system (system absorbs heat);
-w → work is done by the system (system releases heat).
There are 3 types of Standard Enthalpy Changes under the following standard conditions:
M = 1mol/L
P = 1bar = 1atm = 100kPa
T = 298K = 25oC (room temperature)
- Standard Enthalpy Change of a Reaction - ∆Ho or ∆Hro or ∆Ho298
To calculate the standard enthalpy change of a reaction, we use a simple formula:
∆H = Hproducts - Hreactants
Example:
x AB + y CD → z EF + u GH


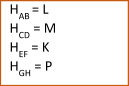
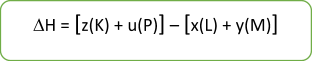
If the resulting ∆H is positive – the reaction is endothermic (heat is absorbed during the reaction)
If the resulting ∆H is negative – the reaction is exothermic (heat is released during the reaction)


- Standard Enthalpy Change of Combustion - ∆HCo
Standard enthalpy change of combustion is the enthalpy change when 1 mole of a substance is completely burnt (combusted) in excess oxygen under standard conditions.
- Standard Enthalpy Change of Formation - ∆Hfo
Standard enthalpy change of formation is the enthalpy change when 1 mole of a substance is formed from free elements in their most stable states under standard conditions.
Let’s consider the following sample problem to better understand the concept of enthalpy change:
Sample Problem:

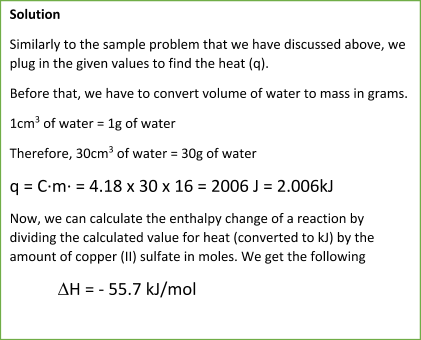
NOTE:
Pay attention to the term exothermic that was provided in the problem. Since the reaction is exothermic, we must put the negative sign next to the calculated enthalpy change value. If you forget to do so, you will lose credits for the problem since the negative and positive signs make a huge difference.
If there is an endothermic reaction, you just put a positive sign to the enthalpy change value.
DO NOT FORGET that heat is measured in Joules while the enthalpy change is measured in kJ/mol. When you calculate the value for the heat, you must convert the value in J to kJ to get the proper result for enthalpy change.
Hess’s Law
Hess’s Law states that the enthalpy change for an overall process is equal to the sum of the enthalpy change of its individual steps.
∆Hoverall = ∆H1 + ∆H2 + … + ∆Hn
The overall enthalpy change is not dependent on the reaction route taken as long as the initial and final conditions are the same.
Summary
| System | Open system – transfer of mass and energy. Closed system – transfer of energy but not massIsolated system – no transfer of mass or energy |
| Exothermic reaction | Heat is released |
| Endothermic reaction | Heat is consumed |
| Heat | The transfer of thermal energy between two borders with different temperatures. q = C∙m∙∆T |
| Calorimeter | Device used for experimental determination of heat change in a system |
| Heat capacity | Quantity of the heat absorbed or released due to the temperature change of 1oC or 1K. C = q∆T , J/oC |
| Specific heat capacity | the amount of heat required to raise the temperature of 1g of a substance by 1oC or 1K. C = qm∆T , J/goC |
| Enthalpy | a quantity used to define the total amount of heat of a system. ∆H = ∆U + P∆V |
| Standard Enthalpy Change of a Reaction | ∆H = Hproducts - Hreactants |
| Hess’s Law | the enthalpy change for an overall process is equal to the sum of the enthalpy change of its individual steps. ∆Hoverall = ∆H1 + ∆H2 + … + ∆Hn |
Frequently Asked Questions
What are endothermic reactions?
These are the reactions in which energy is absorbed during a chemical reaction. The overall energy of the products formed in these reactions is greater than the energy of the reactants. E.g., the melting of ice is an endothermic physical reaction.
What is specific heat capacity and its formula?
Specific heat capacity is the amount of heat (in joules) required to raise the temperature of 1kg of a substance by 1 kelvin. Its unit is J per kg per kelvin. The formula for specific heat capacity is c = Q / (mΔT), where “c” is the specific heat capacity.
What is the enthalpy change for an exothermic reaction?
For an exothermic reaction, the enthalpy change will be negative showing that energy is released during the reaction and the overall energy of products is less than that of reactants. This energy is usually dissipated in the form of heat.
What are two types of systems in thermochemistry?
The two systems in thermochemistry include; the closed system and open system. In a closed system, nothing can enter or leave the system while in open systems, things may enter or leave the system.
References:
Encyclopedia Britannica. n.d. “Calorimeter.” Retrieved from: https://www.britannica.com/technology/belt-drive
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





