Table of Contents
Definition: The mole (n)
The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance.
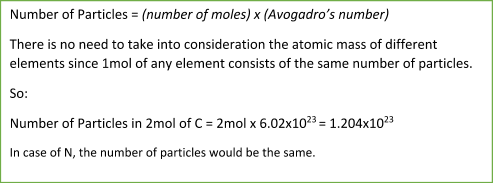
It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons).
6.02x1023 is a unique number, called Avogadro’s number.
Amedeo Avogadro was the first scientist to determine the actual number of particles in a mole. Avogadro’s number is defined as the number of carbon atoms in 12.00g of the pure carbon isotope 12C (with a mass of 12amu).
According to this statement, one mole of any substance (molecule, chemical, book, student, dog, elephant, etc.) consists of 6.02x1023 of that particular thing.
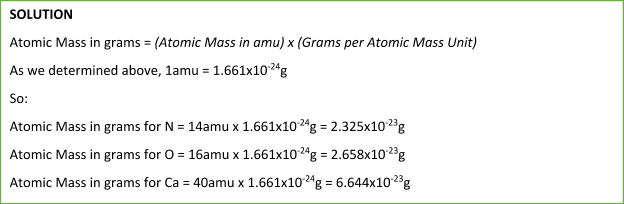
Using Avogadro’s number of 12C isotope with a mass of 12.00g, we can calculate the mass of 1amu in grams:
6.02x1023 C atoms (12amuC atom) = 12g
6.02x1023amu = 1g
Therefore, 1amu = 1.661x10-24g
Using the value of 1amu calculated above (1amu=1.661x10-24), we can calculate the mass of individual atoms in grams.
Read more about the Mole and Equations
Sample Problem:


Molar Mass (M)
Molar Mass (M) is the mass of 1mol of a compound in grams (g/mol, grams per mole). Even though the molar mass of elements has the same value as Relative Atomic Mass (Ar) provided in the periodic table, the units of molar mass are “g/mol,” while the units of atomic mass are “amu.”
Similarly, the molar mass of 1 mole of any element/substance is equal to its Relative Atomic Mass (Ar) or Relative Molecular Mass (Mr) but in units of “g/mol.”
Relative Atomic Mass – abbreviated as Ar. Defined as a mass of one atom of an element
Relative Molecular Mass – abbreviated as Mr. Defined as a mass of 1mol of a molecule (the sum of the relative atomic masses of its constituent elements).
So, the mass of 1mol of an element is equal to its relative atomic mass in grams; and the mass of 1mol of a compound is equal to its relative molecular mass in grams.
Sample Problem:

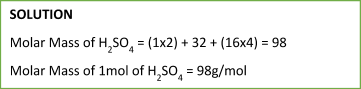
Using the number of moles and the value of the molar mass of a particular substance, we can easily determine the mass:
Sample Problem:

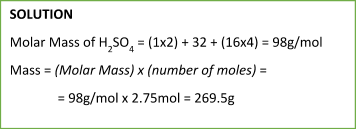
The relations between molar quantities are quite straightforward.
For example, let’s consider atomic hydrogen, H. Formula mass of H is 1amu, and molar mass is 1g/mol. Since there are no coefficients or indexes, we say that there is 1 mol of H atoms.
In the case of molecular hydrogen, H2, we cannot say that there is 1 mol of H atoms, since we have 2 as a subscript, meaning that there are 2 H atoms, therefore 2 moles of H atoms. On the other hand, we can state that there is 1 mole of H2 molecules.
Similarly:
Water – H2O
- 1mol H2O molecules
- 1mol O atoms
- 2mol H atoms
Calcium Bromide – CaBr2
- 1mol CaBr2 molecules
- 1mol Ca atoms
- 2mol Br atoms
To calculate the number of moles of any substance, we can use the following formula:
Number of Moles = massmolecular ( molar) mass
Sample Problem:

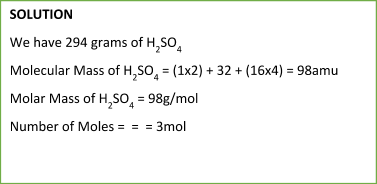

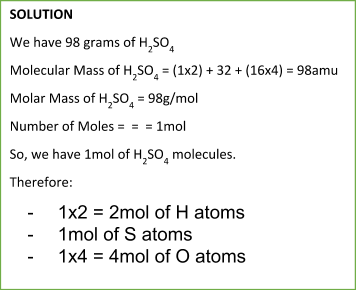

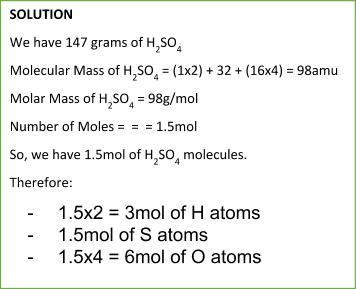
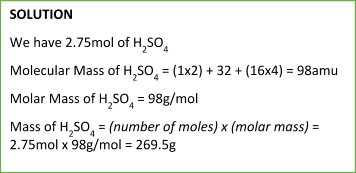
Similarly, we can calculate the mass of a particular substance from a number of moles using the following equation:
Mass of a Substance = (number of moles) x (molecular/molar mass)
Sample Problem:

Molar Volume
In case if a substance is in gaseous form, the volume that 1mol of this gas occupies at room temperature and pressure is defined by the term Molar Volume.
Molar Volume of gases = 24litre/dm3 (at room temperature and pressure)
So, 1mol of CO2 occupies the volume of 24L/dm3
Sample Problem:


We have considered some basic examples so far. Now, let’s take a look at a simple problem that involves a reaction between two compounds.


Sample Problem:
In case if we have the following balanced equation:
2A + B = A2B
If the problem says that the amount of compound A is 2mol, then the amount of the product will be twice as less (1mol).
Summary
It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons).
6.02x1023 is a unique number, called Avogadro’s number.
Molar Mass (M) is the mass of 1mol of a compound in grams (g/mol, grams per mole).
The mole is a quantity used to characterize the amount of substance. Every 1 mol of any substance consists of 6.02x1023 particles of that substance.
To calculate the amount of an element/compound in moles, we just use the simple equation n=m/M, where: n – the amount of substance in moles; m – a mass of a substance in grams; M – the molar weight of a substance in grams per mole.
At first glance, it might seem a little bit confusing to fully understand the idea of the concept. But things get a lot more straightforward as long as you practice and get familiar with common conversions from moles to the number of particles, from grams to moles, etc.
The mole is one of the essential quantities used in various equations in Chemistry. So, make sure that you entirely understand the concept since this is the basis for more advanced topics.
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.
Frequently Asked Questions
What is a mole?
One mole is the no. of particles present in one gram of an element, molecule, or formula unit. For example; the no. of carbon atoms present in 12g of carbon is called one mole. One mole of carbon consists of 6.02 × 1023 carbon atoms.
What is molar volume?
Molar volume defines as; the “volume occupied by the one mole of a substance (element, molecule, ion or formula unit) at standard temperature and pressure (i.e., 273K and 1.0 atm).
Are molar volume and molar mass the same?
No, the molar mass is the sum of protons and neutrons present in an atom while molar volume is the volume occupied by one mole of a substance.
What is Avogadro’s number and what is its value?
Avogadro’s number is the no. of particles present in one mole of a substance whether it is an element, molecule, ion or formula unit. Its value is 6.02 × 1023 and is shown with the symbol NA.





