Table of Contents
Compounds which incorporate carbon-carbon bonds or covalent bonds of carbon with other elements like hydrogen, nitrogen, oxygen, sulfur, phosphorous and halogens, are generally classified as organic compounds. The chemical properties of organic compounds are intricately related to their structures. Hence, the classification of organic compounds according to their structures, is an essential part of understanding the reactivity of these compounds. Keep reading to learn more about structures and reactions of organic compounds.
The organic compounds can be classified into major groups as:
Aliphatic Compounds
Aromatic Compounds
Heterocyclic Compounds
Polymers
Biomolecules
Small Molecules
Fullerenes
Apart from this classification, the reactivity of the organic compounds depends on the functional groups. The functional groups are defined as “specific groups (moieties) of atoms or bonds within molecules that are responsible for the characteristic chemical reactions of those molecules”. These functional groups, along with the class of organic compounds, shape the reaction outcomes.
Out of the many functional groups that can be encountered in various levels of chemistry, the organic chemistry study recognizes some of these as the most common functional groups. These are:
The most common functional groups
Hydrocarbons
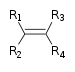
Hydrocarbons consist of only carbon and hydrogen as the reactive sites. These can be further classified as alkane, alkene, alkyne and benzene derivatives. The structures of each of these are given below in sequence.
![]()
![]()
![]()
Alkane alkene alkyne benzene derivatives
Halogen-containing groups
Two main classes of halogen-containing groups exist namely, haloalkanes and haloarenes (aryl halides). The general structure of these are respectively:
![]()
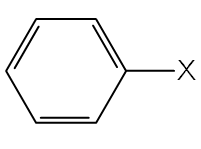
Haloalkanes haloarenes (aryl halides)
Oxygen-containing groups
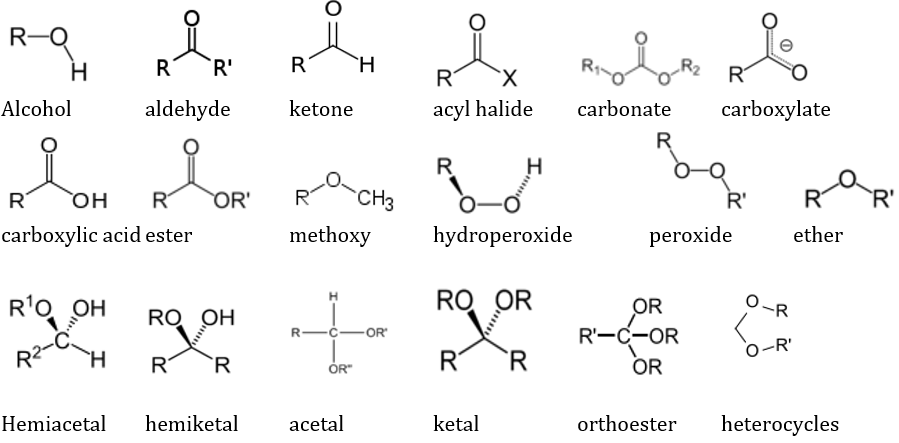
The impact of the second most electronegative element oxygen is clearly visible in different functional groups, depending on the location, bond strength and hybridisation of the covalent bond between C and O. The main classes of O containing groups are: Alcohol, aldehyde, ketone, acyl halide, carbonate, carboxylate, carboxylic acid, ester, methoxy, hydroperoxide, peroxide, ether, hemiacetal, hemiketal, acetal, ketal, orthoester, and heterocycles.
These are sequentially shown below:
Nitrogen-containing groups
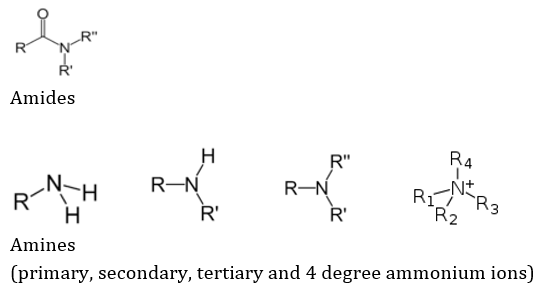
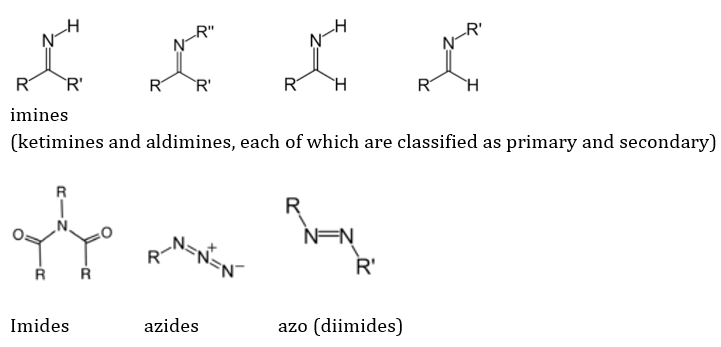
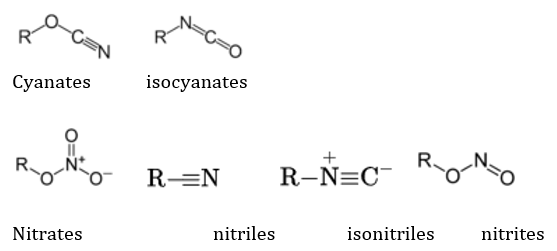
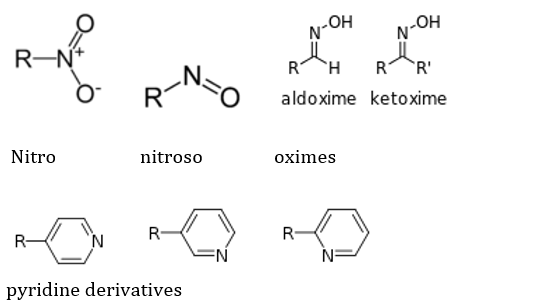
The N containing groups are dependent not only on nitrogen, but occasionally on other atoms like O for their reactivity, but are still classified as such because of the crucial role of N. Some of the N-containing groups are: Amides, Amines (primary, secondary, tertiary and 4 degree ammonium ions), imines (ketimines and aldimines, each of which are classified as primary and secondary), imides, azides, azo (diimides), cyanates (and isocyanates), nitrates, nitriles (and isonitriles), nitrites, nitro, nitroso, oximes and pyridine derivatives. These are represented respectively as follows:
Sulfur, Phosphorous, and Boron containing groups
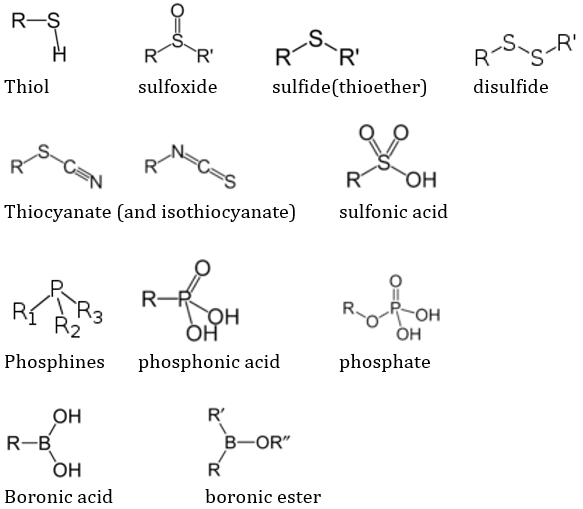
Organic chemistry also includes some of the S, P, and B containing groups. The frequently encountered ones are: thiol, sulfoxide, sulfide(thioether), disulfide, thiocyanate (and isothiocyanate), sulfonic acid, phosphines, phosphonic acid, phosphate, boronic acid and borinic ester.
These are represented sequentially, as follows:
All of these structures alter the course of a reaction in their own way, but still, the organic reactions may be classified into some major categories as follows:
Addition reactions
Elimination reactions
Substitution reactions
Rearrangement reactions
Radical reactions
Oxidation-Reduction reactions
These are characterized as follows:
Addition reactions
The reactions in which all the components of the reactants are added to the C atoms on adjacent sides, in lieu of multiple C-C bonds. No, atoms are released as byproducts.
Example:
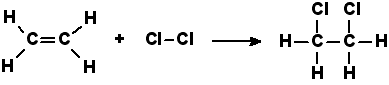
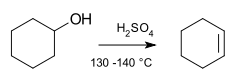
Elimination reactions
These reactions are opposite to addition reactions, in which, the substituents are removed in one or two steps.
Example:
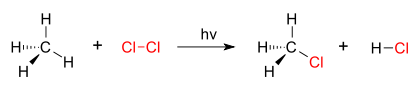
Substitution reactions
This is a type of chemical reaction where one functional group is replaced by another.
Example:
The substitutions reactions may further be divided into
Nucleophilic substitution - a reaction where an electron-rich nucleophile attacks a positively charged centre to replace another group (leaving group). These can be further classified as:
SN1 reactions: which are two-step processes where the first (slow) step is the leaving of the replaceable group and the second step is the fast attack by the nucleophile.
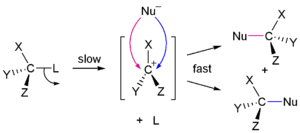
SN2 reactions: which are one-step processes where the attack by the nucleophile & leaving of the replaceable group happens simultaneously.
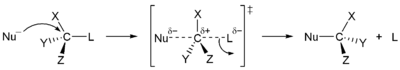
Electrophilic substitution - a reaction where an electrophile replaces another functional group.
Example:
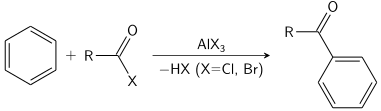
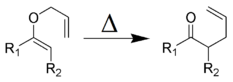
Rearrangement reactions
The reaction of organic compounds where the product formed is a structural isomer of the reactant.
Example: Claisen rearrangement
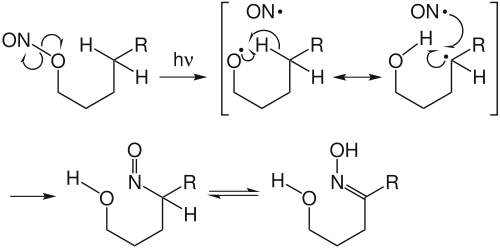
Radical reactions
This class of reactions occurs through the free-radical mechanism by homolytic bond cleavage in presence of heat or light. The three steps of such reactions are initiation, propagation, and termination.
Example: Barton reaction
Oxidation-Reduction Reactions
In organic chemistry, the definition of redox reactions is slightly different from inorganic chemistry, since most of these do not involve actual electron transfer. Hence, the term oxidation means the addition of oxygen and/or loss of hydrogen. The term reduction means the addition of hydrogen or loss of oxygen.
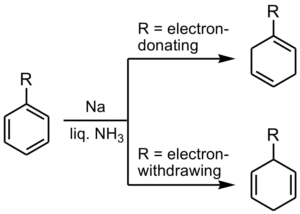
Example: Birch reduction
Read more about Organic Synthesis
Frequently Asked Questions
What are aromatic hydrocarbons?
Hydrocarbons which have cyclic, planer structures and contain one or more benzene rings are known as aromatic hydrocarbons because of the pleasant smell (aroma) of these compounds. E.g., benzene, naphthalene, toluene etc.
What are elimination reactions?
Reactions in which small molecules like H2O, or hydrogen halide i.e., HCl etc. are removed from a compound by either a 2 step or 1 step reaction. E.g., when an alkyl halide is heated in the presence of an alkaline ethanol medium, it eliminates a hydrogen halide forming an alkene.
What are the examples of organic compounds containing oxygen in their functional groups?
Organic compounds containing oxygen in their functional groups are; alcohols, aldehydes, ketones, ethers, esters, peroxide, hemiacetal, hemiketal, acetal, ketal, orthoester, acyl halide etc.
Is cyclohexane an aromatic compound?
Cyclohexane is a saturated, cyclic, planer compound but not an aromatic compound because it does not have any benzene ring in its structure. .





