Table of Contents
Key Facts & Summary for the Structure and Properties of Amines:
- An amine is an organic compounds or a functional group that contains a basic nitrogen atom.
- The nitrogen in an amine has a lone pair (a pair of valence electrons that are not shared with another atom).
- The polar nature of the N-H bond (due to the electronegativity difference of the two atoms) results in the formation of hydrogen bonds .
- High melting and boiling points compared to analogous alkanes.
- High solubility in aqueous media.
- Amines are bases, and their basicity depends on the electronic properties of the substituents, steric hindrance and on the degree of solvation of the protonated amine.
- Primary and secondary amines are also very weak acids.
- Amines are quite reactive due to their basicity as well as their nucleophilicity.
- Keep reading for more facts about the structure and properties of amines
An amine is an organic compound or a functional group that contains a basic nitrogen atom with a lone pair of electrons. They derive from ammonia, in which one or more hydrogen atoms have been replaced by a carbon-containing substituent.
Structure
Amines are classified according to their degree of substitution at the nitrogen atom. An amine contains a nitrogen atom, a lone pair of electrons, and three substituents. An amine with one carbon attached to nitrogen is a primary amine, an amine with two is a secondary amine, and an amine with three is a tertiary amine.
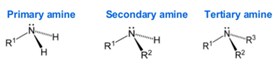
Finally, cyclic amines are those in which the nitrogen has been incorporated into a ring structure, effectively making it either a secondary or tertiary amine.
Read more about the structures and reactions of organic compounds
Physical Properties of Amines
The N-H bond is polar, so amines can create hydrogen bonds. As a consequence, their boiling points are lower than those of the corresponding alcohols, because alcohols creates a stronger net of hydrogen bonds.
Amines are generally soluble in water. If the number of carbon atoms increases, the hydrophobicity of the compound increases too, decreasing the solubility. Amines connected to an alkyl chain are called aliphatic amines and display solubility in organic polar solvents. Aromatic amines are amines participating in a conjugated ring. They donate their lone pair of electrons into the benzene ring, and thus their ability to create hydrogen bonding decreases. This results in a decrease in their solubility in water and high boiling points.
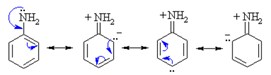
Acidity and Alkalinity of Amines
Amines are bases. A base is either a substance which combines with hydrogen ions (Bronsted-Lowry theory) or an electron pair donor according to the Lewis theory.
In an amine, one or more of the hydrogen atoms in ammonia has been replaced by a hydrocarbon group. Replacing the hydrogens still leaves the lone pair on the nitrogen, therefore it behaves much the same as ammonia in all cases where the lone pair is involved.
In a water solution of diluted acid, the ammonia takes a hydrogen ion (a proton) from a hydroxonium ion.
NH3(aq)+H3O+(aq)→NH+4+H2O(l)
In water, the ammonia is only a weak base. The reaction is reversible, with the great majority of the ammonia present as free ammonia rather than ammonium ions.
NH3 + H2O ⇌ NH+4 + OH−
The presence of the hydroxide ions from this reaction makes the solution alkaline.
The amine still contains the nitrogen lone pair, and behaves similarly. For example, with ethylamine, ethylammonium ions and hydroxide ions are produced:
CH3CH2NH2 +H2O ⇌ CH3CH2NH+3 +OH−
There is, however, a difference in the position of equilibrium. Amines are usually stronger bases than ammonia.
Amine basicity depends on the electronic properties of the substituents. Alkyl groups enhance the basicity while aryl groups diminish it. The delocalisation of the lone pair of electrons on nitrogen into a ring results in a decreased basicity. Furthermore steric hindrance and the degree of solvation of the protonated amine influence the basicity too.
Primary and secondary amines are also very weak acids (ammonia has a pKa = 34). The same factors that decreased the basicity of amines increase their acidity.
Amine Reactivity
Amines are quite reactive due to their basicity as well as their nucleophilicity.
A nucleophile is an atom or a part of a molecule which is attracted and reacts with a positive part of another molecule or ion. The lone pair of electrons generate a high density of negative charge on the nitrogen atom. This part is attracted to positive parts of other molecules or ions.
Most primary amines are good ligands and react with metal ions to yield coordination complexes. One of the most important reactions for amines is their formation of imines, or organic compounds where nitrogen participates in a double bond, upon reacting with ketones or aldehydes.
Applications of Amines
Amines are ubiquitous in biology. Many important molecules are amine-based, such as neurotransmitters and amino acids. Their applications in the world include being starting material for dyes and models for drug design. They are also used for gas treatment, such as removing CO2 from combustion gases.
Frequently Asked Questions
What are amines?
Amines are the organic compounds derived from ammonia that have nitrogen containing a lone pair of electrons.
What are the physical properties of amines?
Amines can create hydrogen bonds due to the polarity of the N-H bond. They are water-soluble, but the solubility decreases when the number of carbon atoms increases.
What are the uses of amines?
Amines are used in the preparation of dyes and drugs. They are also used in gas treatment, such as removing CO2 in combustion gases.
Why do amines have basic character?
Amine has a lone pair of electrons that can be donated easily to an H+ ion from acids, so they are basic.
References and further readings:
https://www.youtube.com/watch?v=Kpov3GS6tjM
https://courses.lumenlearning.com/introchem/chapter/amines/
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch22/ch22-1.html
“Organic chemistry”, Francis A. Carey, ISBN 0-07-117499-0





