Table of Contents
Introduction to States of Matter
The quest to know the nature and functioning of matter has been the endeavour of scientists since time immemorial. Anything that takes up some space and has mass has typically been defined as matter. Accordingly, the three most common states of matter found on earth are solid, liquid, and gas.
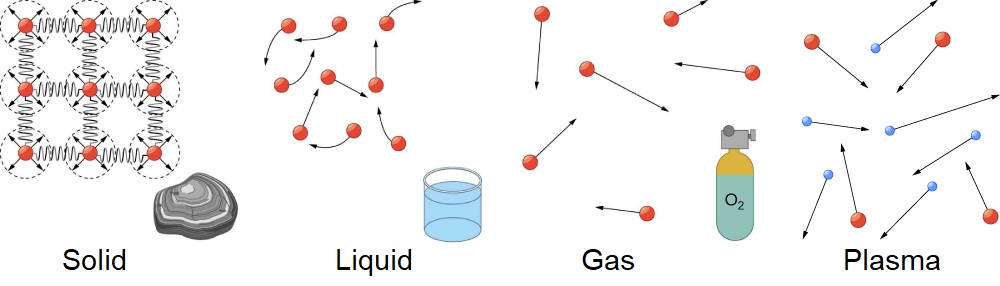
A solid is, in most cases, rigid and holds a definite shape. Exceptions include gums that can be moulded into different shapes and even pressed into different sizes. A liquid typically flows and, when inside a contained, takes its shape, although the upper surface remains flat or slightly curved. This, however, is the case when a liquid is under the influence of gravity. In zero gravity, liquids assume a spherical shape. Under ordinary conditions prevalent on earth, both liquids and solids occupy volumes almost independent of pressure. Gases, however, are free-flowing in nature and tend to take up both the volume and shape of the container. Under conditions when no container is present, gases can move freely and have different volumes and pressures according to the surroundings. Apart from these states, another state of matter is found naturally inside the interior of stars. This state is called plasma. A plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles. The simultaneous occurrence of two or more of the three common states of matter is quite possible in nature and man-made circumstances. One of the most suitable substances that show this phenomenon is water. It can simultaneously exist in liquid water, solid ice, and gaseous water vapour.
The existence of matter is governed by the law of conservation of matter which states that – there is no detectable change in the total quantity of matter present when matter converts from one type to another (a chemical change) or changes among solid, liquid, or gaseous states (a physical change).
Classification of Matter
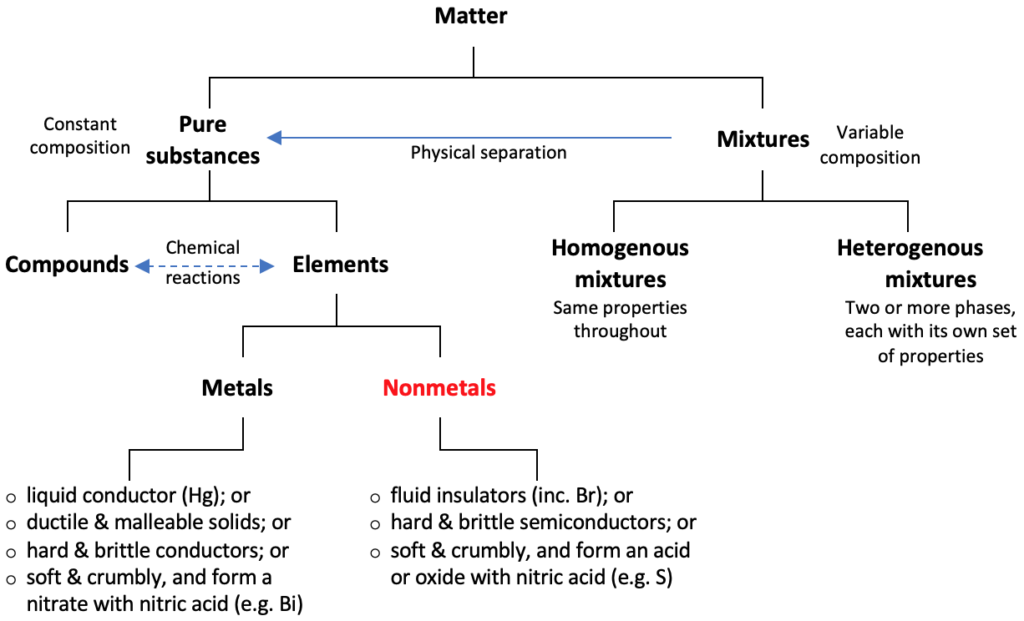
Classification of matter can sometimes be tricky because matter can consist of different elements or compounds or mixtures, or all of these. Therefore, one aspect of classifying matter is to classify them into pure and impure substances.
A pure substance has a constant proportion of constituents that cannot change by any physical activity. However, it can change via chemical reactions, in which case, the state of matter may or may not change.
Read more about Mass Spectra and IR Spectroscopy
Properties of Matter
Solids
- Structural rigidity and resistance to changes of shape or volume.
- A solid object does not flow to take on the shape of its container, nor does it expands to fill the entire volume available to it.
- The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary water ice) or irregularly (an amorphous solid such as common window glass).
- Sublimation – some solids transform into the corresponding gaseous phases under normal temperature and pressure.
Liquids
- Capillary action – the ascension of liquids through slim tubes, cylinders, or permeable substances due to adhesive and cohesive forces interacting between the liquid and the surface.
- Surface tension – the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
- Vapour pressure – the pressure of a vapour in thermodynamic equilibrium with its liquid (condensed) phase in a closed container.
- Viscosity – a type of bulk property defined as a liquid’s resistance to flow.
- Wettability – the ability of a liquid to spread over a solid in contact.
- Solvation – typically, all liquids can act as solvents to dissolve either solids or liquids or gases or all, depending on specific properties.
Gases
- Pressure – gases exert a force on the surface in contact, which is called gas pressure.
- Gases have no definite shape, size, or volume.
- Gases tend to flow from a high-pressure region to an area of low pressure.
- Gases expand to occupy the entire volume of the container.
Plasma
- Contain a mixture of charged species, including ions and atoms.
- Have no definite shape, size, or volume.
- Have a very short-lived span.
The Transition of State of Matter
Chemical Changes
The ability or the inability to change one type of matter into another type is a chemical property. Those materials that can undergo such changes are called chemically reactive, and those which cannot are chemically inert. A chemical change always produces one or more types of matter that differ from the matter present before the change.
Physical Changes
These kinds of changes do not alter the chemical properties of the matter; in other words, the originality of matter is preserved. However, physical changes can be quite drastic and affect the functioning of a material. A classic example is water, which has different uses and properties in different states.
Checkpoints for the Transition of States of Matter
Matter can change from one state to another following certain checkpoints, which can be defined as –
Melting point – the temperature at which a solid turns into a liquid.
Boiling point – the temperature at which a vapour of liquid equals the atmospheric pressure.
Freezing point – the temperature at which a liquid turns into a solid.
Evaporation point – the temperature at which a liquid turns into a gas.
Condensation point – the temperature at which gas turns into a liquid.
Effect of Temperature and Pressure on States of Matter
The states of matter owe their forms to the prevalent temperature and pressure. On the earth’s surface, the pressure is more or less similar, and hence the pressure has been denominated at a value of 1 atm. This is the pressure exerted by the air on the earth’s surface, or in other words, the combined pressure of all gases on earth. However, pressure in the case of gases can be classified into two types. Thermodynamic pressure is the pressure exerted by the walls of the container on the gas. Changing this pressure can lead to condensation of the gas into the corresponding liquid. Another type of pressure specific to the gases is kinetic pressure. This is the pressure exerted by the gaseous atoms/molecules on the surface in contact. It is this pressure that contributes to the atmospheric pressure.
For liquids, its vapours just above the surface give rise to a vapour pressure for liquids. Typically, it is much harder to compress a liquid into a solid than to compress gas into a liquid by applying pressure. Solids typically break under pressure since they can no further be compressed.
An increase in temperature makes the solids convert to liquids and liquids to gases, although direct sublimation of solids is also possible.
The In-Between Cases – Matter that cut across the Classifications
There are some physically observable phenomena that suggest flexibility in the classification of states of matter is necessary.
- Gums and adhesives – These are typically mouldable and can be given different shapes and sometimes even flow, and yet occupy a definite shape without any container.
- Fire – for a long time, the state of fire has been speculative, and it can be thought of as being close to plasma.
- Glass – although glass is thought of generally as an amorphous solid, it can very well be classified as a supercooled liquid.
- Bose-Einstein Condensate – a superfluid in a gaseous state made of atoms that have been cooled at temperatures nearing zero
The Information about the States of Matter can be summarized as follows –
- Matter typically exists in 4 states – solids, liquids, gases, and plasma
- The states of matter are interchangeable with or without changing the chemical properties.
- Temperature and pressure have a great impact on states of matter.
- There are materials that are hard to be classified into any one state of matter because their properties are cut across the states.
Frequently Asked Questions
What are the different properties of liquids?
Liquids are incompressible, have fixed volumes and can flow from higher pressure to low-pressure areas. Liquids also show the phenomena of; capillary action, viscosity, solvation, surface tension, wettability, vapuor pressure and evaporation.
Which state of matter is considered to be the fourth stage of matter?
Plasma is considered to be the fourth state of matter. It consists of charged particles i.e., negative and positive ions and electrons. It has a very short life and exists only at very high temperatures.
What is the transition state of matter?
A transition state is one in which two states of matter coexist such as solid and liquid or liquid and gas. It exists at a certain temperature called transition temperature. For example, the transition temperature of the water is 0℃ at which it can exist in both solid and liquid forms.
What is sublimation?
The process of conversion of solids into gases without going into a liquid state is called sublimation. Different solids show the phenomenon of sublimation i.e., iodine etc.
References
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Phase_Transitions/Fundamentals_of_Phase_Transitions
- https://openstax.org/books/chemistry-2e/pages/10-5-the-solid-state-of-matter
- https://openstax.org/books/college-physics-ap-courses/pages/11-1-what-is-a-fluid





