Table of Contents
Key Facts & Summary:
- Spectroscopy deals with the production, measurement, and interpretation of spectra arising from the interaction of electromagnetic radiation with matter.
- The electromagnetic wave can be characterized by its frequency of oscillation or its wavelength.
- When a molecule is exposed to electromagnetic radiation, it may absorb a photon.
- Spectrometers are instruments designed for measuring a specific photon interaction in order to obtain specific information about the studied molecule.
- The data obtained from a spectroscopic measurements is called “spectrum”.
- The several spectroscopic techniques can be classified in several ways: by the type of radiative energy or by the nature of the interaction.
- A spectroscopic measurements can be used for measure the amount of a specific substance in a sample (quantitative analysis) or even just to identify an unknown sample (qualitative analysis).
Spectroscopy deals with the production, measurement, and interpretation of spectra arising from the interaction of electromagnetic radiation with matter.
Light waves and other types of energy (radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays) that radiate from where they're produced are called electromagnetic radiation. In physics, electromagnetic radiation refers to the waves of the electromagnetic field, propagating through space. Electromagnetic waves are waves that contain an electric field (E) and a magnetic field (M) and carry energy. They travel at the speed of light which, in a vacuum, is commonly denoted c.
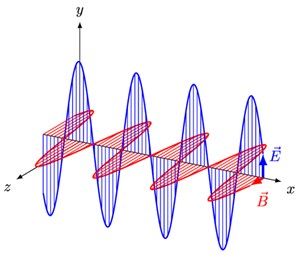
In homogeneous, isotropic media, the oscillations of the two fields are perpendicular to each other and perpendicular to the direction of energy and wave propagation, forming a transverse wave.
The electromagnetic wave can be characterized by its frequency of oscillation or its wavelength. Frequency is the number of oscillations per time and wavelength is the distance over which wave's peak repeats.
Electromagnetic waves of different frequencies are called by different names since they have different sources and effects on matter. In order of increasing frequency and decreasing wavelength: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays.
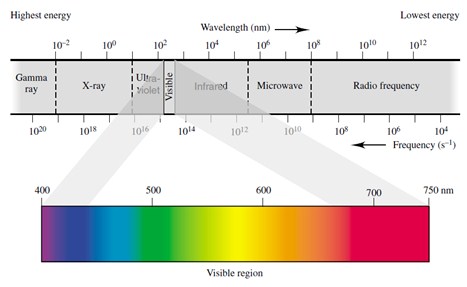
It is interesting to notice that the frequency is inversely proportional to wavelength: the greater the frequency is, the shorter the wavelength. The Energy is directly proportional to frequency: electromagnetic radiation of higher frequency are more energetic than radiation of lower frequency.
Gamma ray and X-ray photons bring very high energy. Radio waves are of relatively low energy. Ultraviolet radiation has higher energy than the violet end of visible light. Infrared radiation has lower energy than the red end of visible light.
When a molecule is exposed to electromagnetic radiation, absorption can occur: the molecule may absorb a photon and increase its energy by the energy of the photon. The photon need to have specific frequency suitable for the molecules in order to be absorbed. The photon energies absorbed by a molecule depend on the molecular structure. Spectroscopic techniques measure these energies. Spectrometers are instruments designed for measure a specific photon interaction in order to obtain specific information about the molecule studied.
In general, spectrometer has an energy source (commonly a laser, but this could be an ion source or radiation source) and a device for measuring the change in the energy source after it has interacted with the sample.
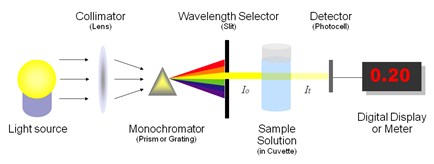
The data obtained from a spectroscopic measurement is called a spectrum. A spectrum is a plot of the intensity of energy detected versus the wavelength (or mass or momentum or frequency, etc.) of the energy.
A spectrum can be used to obtain information about atomic and molecular energy levels, molecular geometries, interactions of molecules, chemical bonds.
A spectroscopic measurement can be used for measure the amount of a specific substance in a sample (quantitative analysis) or even just to identify an unknown sample (qualitative analysis).
Read more about infrared spectroscopy
Classification of methods
Spectroscopy is a broad field, thus many sub-disciplines exist. The several spectroscopic techniques can be classified in several ways: by the type of radiative energy or by the nature of the interaction.
There are multiple ways to classify types of spectroscopy. The techniques may be grouped according to the type of radiative energy (e.g., electromagnetic radiation, acoustic pressure waves, particles such as electrons), the type of material being studied (e.g., atoms, crystals, molecules, atomic nuclei), the interaction between the material and the energy (e.g., emission, absorption, elastic scattering), or by specific applications (e.g., Fourier transform spectroscopy, circular dichroism spectroscopy).
Examples
UV-visible absorption spectroscopy
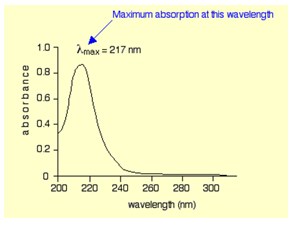
Ultraviolet-visible spectroscopy refers to absorption or reflectance of the light in the ultraviolet-visible spectral region. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene. Absorbance (on the vertical axis) is a measure of the amount of light absorbed. The higher the value, the more of a particular wavelength is being absorbed.
The infrared absorption spectrum of a substance is sometimes called its molecular fingerprint. Although frequently used to identify materials, infrared spectroscopy also may be used to quantify the number of absorbing molecules.
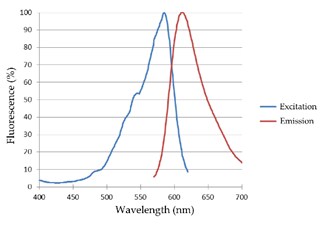
Fluorescence spectroscopy
Fluorescence is the phenomenon where a molecule absorbs light and then emits light. The emission will be at longer wavelengths. This is a popular method due to its high levels of sensitivity, simplicity, and specificity.
Fluorescence spectroscopy is a spectroscopy method used to analyze the fluorescence properties of a sample by determining the concentration of an analyte in a sample. This technique is widely used for measuring compounds in a solution, and it is a relatively easy method to perform.
Frequently Asked Questions
What is spectroscopy?
Spectroscopy deals with the spectra obtained after the interaction of electromagnetic rays with matter. It deals with the emission and absorption of the different types of radiation by matter.
What are the different types of spectroscopy?
Different types of spectroscopy include fluorescence, UV- visible absorption, infrared, and X-ray spectroscopy.
What is fluorescence spectroscopy?
Fluorescence spectroscopy is a procedure that uses a light beam to excite the electrons in molecules and cause them to emit light rays. That light is projected towards a filter and onto a detector to identify the molecule and changes induced in the molecule.
What is UV-visible absorption spectroscopy?
Ultraviolet and visible absorption spectroscopy measures the attenuation of the light beam when it passes through a sample or after reflection from the sample surface.
References and further readings:
https://www.youtube.com/watch?v=dkARLSQWHH8
http://www.rsc.org/learn-chemistry/collections/spectroscopy/introduction#Introduction





