Table of Contents
A crucial factor in understanding the chemical reactions is the knowledge of the molecular structure of the various chemical species. The geometry of the molecules decides the fate of a reaction to a large extent. Although defining the structure of a large molecule according to any fixed geometric shape is not practical, the same can be easily done for smaller molecules and ions. To understand the structural geometry in simplistic terms, the usage of three theories are widespread. Keep reading to learn more about shapes of molecules and ions.
Valence-shell electron-pair repulsion (VSEPR) theory
In the molecules having a p-block element as the central atom, the shape of the molecules get decided by the number of valence electrons present on the central atom. The main postulates of this theory are:
- In a molecule EXn having E–X single bonds, each pair of valence shell electrons on the central atom E are of stereochemical significance, and the molecular shape is determined by the repulsion among those.
Maximum repulsion ensures a geometry where the repelling electron pairs are at the farthest from each other.
- The inter-electronic repulsive forces decrease following the sequence:
lone pair–lone pair> lone pair–bonding pair> bonding pair–bonding pair.
- When there are multiple bonds between E and X, inter-electronic repulsions decrease in the order:
triple bond–single bond> double bond–single bond> single bond–single bond.
- Repulsions amongst the bonding pairs in EXn is subject to the difference in the electronegativities of E and X. More the electronegativity of X, less is the inter-electronic repulsion.
Some key points are to be noted while using the VSEPR theory for structure prediction.
- There is a distinction between the “geometry” and “shape” of a molecule/ ion. Geometry includes the lone pairs while the shape includes only the bonding pairs.
For example, XeF2 has a parent trigonal bipyramidal geometry in which the 3 lone pairs occupy the equatorial plane in an attempt to minimize the lp-lp interaction (see rule 2). As a result, the shape becomes linear.
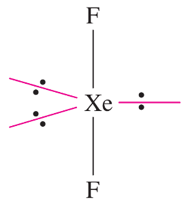
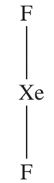
Read more about the shapes of molecules
- This theory works best when dealing with p-block element halides or other simple molecules which do not contain bulky groups. The steric effect of the bulky groups is not considered.
- VSEPR is not applicable to the compounds of d-block elements.
The general outcome of the arrangement f electrons pairs and their effect on the shapes of molecules/ ions is represented below:
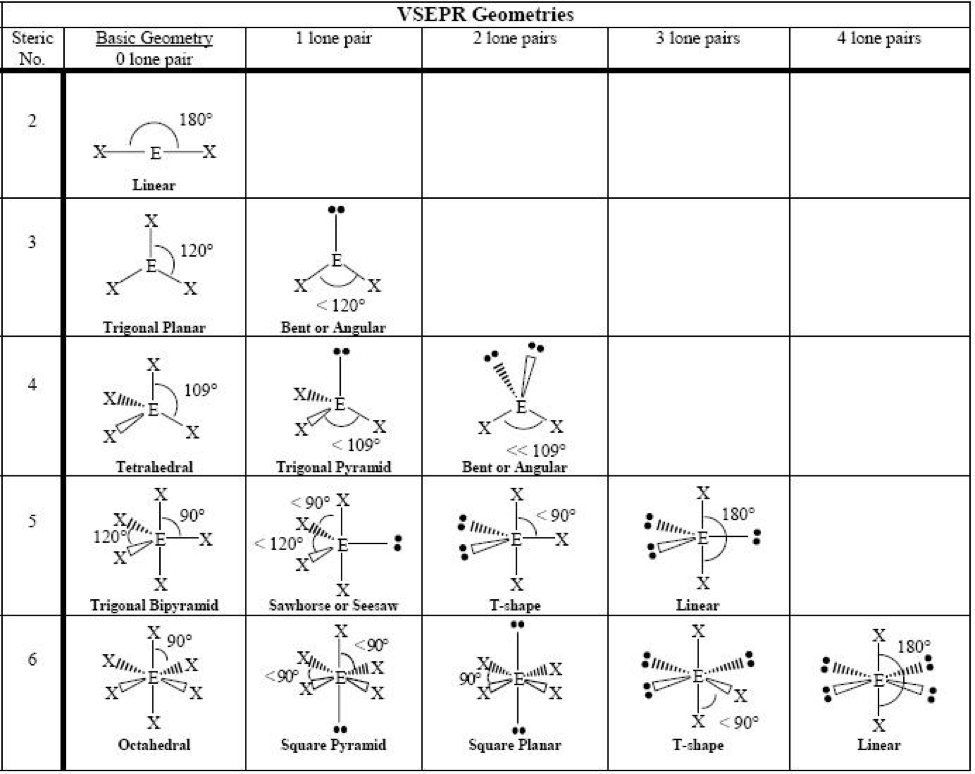
The Valence Bond (VB) Theory
The inclusion of d and later orbitals is crucial to the accuracy in prediction of structures for a wide range of compounds including the coordination complexes and many types of organic compounds. An effective way to accomplish this task is using the Valence Bond (VB) Theory. It is based on the knowledge of the shapes of different atomic orbitals and their mixing to deliver “hybrid” orbitals. Hybridization, hence, is the pivotal point in VB theory.
The important points needed for prediction of structure of molecules/ions by VB theory are:
- Hybrid orbitals are formed by overlap of atomic orbitals. The number of hybrid orbitals formed is equal to that of the atomic orbitals mixed.
- Hybridization always starts from an s orbital, followed by p orbitals, and if needed, d orbitals are included.
- If, in the atomic orbitals there is more than 1 unpaired electron, multiple bonds can be formed but electrons paired in the valence shell are not included therein.
- Directionality is present in covalent bond and it is parallel to the space of overlapping between the atomic orbitals.
- The pattern of overlapping, ensures two kinds of covalent bonds namely sigma and pi bond. A sigma bond is formed by front-on overlap of atomic orbitals while pi bonds are formed by side on overlap.
- The parent geometry follows the order:
sp linear
sp2 trigonal planar
sp3 tetrahedral
sp3d trigonal bipyramidal (dz2 orbital takes part in it)
sp3d square-based pyramidal (dz2-y2 orbital takes part in it)
sp3d2 octahedral
sp2d square planar
The shape of the molecule/ ion depends on the number of lone pairs present.
Since it is a theory, it has some drawback, most notably in the prediction of para/diamagnetic behaviour of molecules. It also suffers from the inability to account for the tetra-valency of carbon. The initial VB theory also considered electron pairs as localized. However, modern VB theory incorporates the idea of delocalized electrons.
Molecular Orbital (MO) Theory
MO theory is one of the most rigorous and successful theories in analyzing the nature of chemical bonds and the resulting molecular structure. Although the quantum mechanical basis does provide a solid foundation to this theory, the same also makes it difficult to understand the mathematically derived structural intricacies even for small molecules. Essentially, the procedure followed for the combination of atomic orbitals to form MO, is called Linear Combination of Atomic Orbitals (LCAO) and the resulting MOs are classified as bonding, antibonding and non-bonding MOs. Some of the important postulates of MOT are:
- Total number of MOs formed, must be equal to that of AO combined to make them.
- BondingMOs have lower energy than the AO forming those.
- AntibondingMOs have higher energy than the AO forming those.
- The MOs are filled up according to the Pauli’s exclusion principle and Hund’s rule.
- Atomic orbitals having similar energies only, combine to form MOs.
In the MO theory, the bond order is the parameter to understand the strength of a chemical bond. The bond order is defined as:
Bond order = half of the difference in the number of bonding and antibonding electrons.
A bond order of 1 indicates a single bond, 2 indicates a double bond, 3 indicates a triple bond while fractional bond orders indicate intermediately strong bonds.
The strength of a bond is directly proportional to the bond order while the length is inversely proportional to it.
The MO theory can also accurately predict the magnetic behavior of a molecule/ion.
An example is given below:
The MO diagram for diatomic oxygen molecule, O2
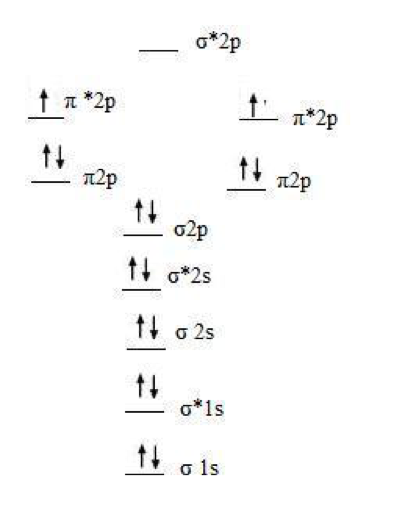
- Here, Bond Order = 1/2(10 - 6) = 2
- It explains the double bonded molecule and its stability.
- The 2 unpaired electrons explain the experimentally observed paramagnetic behaviour.
Frequently Asked Questions
What are lone pairs and bond pairs?
Pair of electrons that take part in bonding is known as bond pairs while those which do not take part in bonding are known as lone pairs. Nitrogen has three lone pairs in its valence shell.
What is the order of inter-electronic repulsive force according to VSEPR theory?
According to the VSEPR (valence shell electron pair repulsion) theory, the order of inter electronic repulsive forces is: “Bond pair-bond pair < bond pair-lone pair < lone pair-lone pair”.
What are the shapes of sp3, sp2 and sp hybridized compounds?
The shape of the molecule in sp3 hybridization is tetrahedral (e.g., ethane). In sp2 hybridization, it is trigonal planer (e.g., ethene) and in sp hybridization, the shape is linear (e.g., ethyne).
What is bond order?
The number of bonding pairs of electrons between two atoms is termed as bond order. For a single covalent bond, the bond order is 1 while in double and triple bonds, the bond order is 2 and 3 respectively. E.g., the bond order of N2 is 3.
References:
1: Inorganic Chemistry by Catherine E Housecroft & Allan G Sharpe, 2nd Edn, Pearson Education Ltd. 2005
2.Chem Libretext https://chem.libretexts.org/
3.https://chem.libretexts.org/





