Table of Contents
Summary
- Chloroalkanes are organic compounds containing carbon and chlorine atoms.
- These organic compounds pose serious threat to our planet.
- They are responsible for ozone depletion.
- The chemical reaction for ozone depletion is catalyzed by UV radiations.
- Comprehensive efforts are in place now to combat emission of these chloroalkanes
- The Montreal protocol has been a great effort to cut down the usage of these compounds.
- Keep reading to learn more about the role of chloroalkanes in ozone layer depletion.
What are chloroalkanes?
Chloroalkanes are groups of alkanes containing chloro groups attached to the carbon atom. They may be mono, di, tri, or tetrachloro substituted compounds. In simple words, we call them alkyl halides. Their simple chemical structure can be shown as:

These organic compounds undergo a wide range of chemical reactions with other reagents. For example, alkyl halides can follow nucleophilic substitution reactions to make series of other organic compounds. Another good example is the Grignard reagent employed to make carbon-carbon bonds and increase the chain length.
Haloalkanes in general and chloroalkanes, in particular, gained tremendous attention during the last three decades. They are held responsible for the depletion of the ozone layer in our atmosphere. This article highlights some of the features of chloroalkanes and their role in ozone depletion.
Read more about the Basic Concepts of Organic Chemistry
The atmosphere of our earth
Our Earth has various layers of gases that surround the planet earth. They are actually a mixture of gases known as air. Collectively these layers are called the atmosphere of the earth. These different layers are illustrated below

The first layer of the troposphere contains the bulk of the mass of our atmosphere. The second is the stratosphere where ozone depletion takes place. This is the mainly important layer where most of the light-induced chemical reactions to take place. The occurrence of these chemical reactions and the presence of ozone is the important point of discussion for global warming, climate change, and ultraviolet layer dangers.
Ozone and ozone layer

Ozone is a triatomic molecule of oxygen with the formula of O3. It is the most important component of the stratosphere where it is responsible to absorb harmful ultraviolet (UV) radiation of the sun. Thus ozone plays a vital role in protecting our planet from dangerous radiation coming from the sun. In the atmosphere, it is made by dioxygen (O2) by the action of UV light and electrical discharges within the atmosphere. Its formation is an endothermic reaction. Ozone is a powerful oxidizing agent and may react with various inorganic and organic substances. Besides its role in atmospheric science, ozone can be used as a chemical reagent in synthesis, potable water purification, as a disinfectant in sewage treatment, and for the bleaching of natural fibers. It is a much more powerful oxidizing agent than ordinary oxygen (O2).
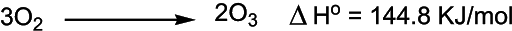
Ozone came to attention first time in the 1840s. Later on, its role in atmospheric science came to light. Measurement of ozone in the atmosphere started in the early 1920s. Its concentration in the atmosphere is reported by using Dobson units or point measurements are reported in parts per billion (ppb). The stratosphere or ozone layer sits around 50km above the earth's surface and though it is called the ozone layer, the concentration of ozone is significantly low. Mostly oxygen is present which under UV radiations is converted to ozone.
Importance of ozone layer & its depletion
Ozone has a quite a strange and confusing role in the atmosphere. In the lower layer of the atmosphere (troposphere), ozone is actually a pollutant and may cause smog formation posing a significant danger to humans and other living animals. If present in a concentration above 0.1ppm level, it may cause serious respiratory problems to humans. However, while this ozone is a pollutant at ground level, the same ozone is beneficial at upper levels of the stratosphere where it is responsible for capturing a major part of the sun’s ultraviolet radiation and preventing this radiation from reaching the earth's surface. Hence it plays a fundamental part in our life on earth. Without a protective ozone layer in the atmosphere, life, animals, and plants could not exist upon the earth.
With all its importance for us and our amazing planet, the ozone layer has been under threat. In the 1970s, the first study highlighted the decreased concentration of ozone in the stratosphere. This decrease in the amount of ozone in the atmosphere is called ozone depletion. During that time, halogenated compounds particularly chloromethane, fluoromethane, and other chlorofluoro hydrocarbons were described as responsible for this ozone depletion. In the early twentieth century, these chlorinated (or halogenated) alkanes were used for refrigeration and air conditioning systems. These compounds are known to be volatile and chemically inert and due to these characteristics, these chlorofluoro carbon compounds (CFCs) had been widely used.
Role of chloroalkanes in ozone depletion
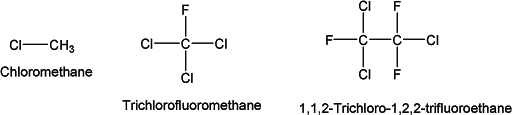
Chloroalkanes are discharged into the atmosphere because of their continuous use in organic synthesis and other industrial processes. They are hydrocarbons containing carbon, hydrogen, chlorine (or fluorine or bromine for other halogenated hydrocarbons). The alkyl chain mainly comes from paraffin and small molecules are made using methane, ethane, or propane. Some of the commonly known chloroalkanes are
The industrial usage of these halogenated compounds has been found as a source of chlorine in the atmosphere. Interestingly, very little chlorine exists naturally in the atmosphere. However, the industrial usage of these chlorinated alkanes can introduce chlorine into the atmosphere. The ultraviolet radiation at this altitude breaks down these compounds, thus generating highly reactive chlorine atoms. These chlorine atoms may react with ozone through a variety of catalytic reactions. For example, a chlorine atom may react with an ozone molecule (O3), taking an oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (O2). The ClO can then react with a second molecule of ozone, releasing the chlorine atom and yielding two molecules of oxygen. This reaction can be presented as
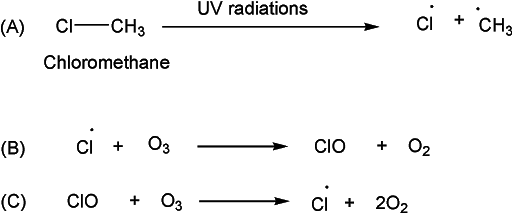
The reactive chlorine atom formed in step (B) is regenerated and thus available to enter into another cycle. The overall impact is a decrease in the amount of ozone. The reaction scheme above is a simple illustration of complex and complicated chemical reactions taking place in the atmosphere. Researchers have discovered more complicated mechanisms that lead to ozone destruction.
A single chlorine atom is able to react with thousands of ozone molecules. This fact and the amount of chlorine continuously being released into the atmosphere yearly demonstrate the danger to the ozone layer and our planet. Eventually, this chlorine has the potential to destroy large amounts of ozone. This has indeed been observed, especially over Antarctica. As a consequence, levels of harmful ultraviolet radiation have increased.
Impact on humans
Ozone depletion has posed serious concerns to the human race and other living organisms (both animals & plants). Increased UV radiation may influence human health, both positively (including production of vitamin D) and negatively (including sunburn, skin cancer, and cataracts). We all know that increased exposure to harmful UV radiations may increase the chances of skin cancer. The mechanism involves the degradation of DNA.
Melanoma is another form of skin cancer. It is far less in frequency, however, more dangerous. The correlation of melanoma to UV radiation is difficult to establish, however, the studies have reported that both UVA and UVB radiation are involved in accelerating this problem.
Increased UV radiations are also hammering animal lives. Recently British scientists have reported sun damage to whales in the Pacific coastal area of the USA. They blame ozone depletion for this sun damage. Similarly, crops and plants are under threat of increased UV exposure. Economical crops require microorganisms to survive and offer production. However some microorganisms, e.g. bacteria are very sensitive to UV radiation.
In short, ozone depletion is posing a serious threat to the whole ecosystem of our planet. Rapid & uncontrolled industrialization is to blame for this. Humans are producing significant volumes of dangerous chloroalkanes and causing massive damage to our planet.
Where do we stand now?
The seriousness of ozone depletion has made headlines across the globe. Public awareness has been raised and pressure has been on politicians and policymakers to design a strategy for this problem. Montreal protocol has been a significant achievement in this regard. The agreement was signed in 1987. It was agreed to phase out halogenated alkanes to cut down chlorine emission. Results demonstrate that this has been a successful agreement. Since then ozone depletion in the atmosphere has decreased. Moreover, in 2019, NASA announced the "ozone hole" was the smallest ever since it was first discovered in 1982. We hope that the ozone layer repairs and recovers with the decreased use of chloroalkanes.
Frequently Asked Questions
Does chloroform cause ozone depletion?
Yes, methyl chloroform is one of the major ozone-depleting agents and poses a serious threat to ozone recovery.
How are chloroalkanes harmful to the ozone layer?
These gases rise slowly to the stratosphere of the atmosphere and produce reactive chlorine radicals that react with ozone and destroy it.
How many molecules of ozone are destroyed by one reactive chlorine radical?
The chlorine radical is regenerated while reacting with ozone. In this way, it can damage thousands of ozone molecules, converting them to molecular oxygen.
What are the effects of ozone depletion?
Ozone depletion allows ultraviolet rays of the sun to reach the earth. Increased UV exposure increases the risk of skin burns, skin cancer, cataracts, and other malformations.
Reference
- Kirschner KM. Ozone, in "Ullmann's encyclopedia of industrial chemistry." (1988).
- Chlorofluorocarbons and Ozone Depletion, A National Historic Chemical Landmark: American Chemical Society page: Accessed on 10-11-19. https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/cfcs-ozone.html
- The Ozone Hole, accessed online: 10-11-19. http://www.theozonehole.com/index.htm
- Cary, A, F. Organic Chemistry, 3rd edition, (1996).
- Volhardt K, P, C. Organic Chemistry, (1987).
- Ozone Hole Above Antarctica Shrinks to Smallest Size on Record: Accessed online on 10-11-19 https://www.wsj.com/articles/ozone-hole-above-antarctica-shrinks-to-smallest-size-on-record-11571847944
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





