Table of Contents
Summary
- Alcohols are organic compounds containing hydroxyl group attached to carbon chain.
- They undergo some of the most important chemical reactions.
- The chemical reactivity of alcohols is related the presence of hydroxyl group.
- Three reactive sites for alcohol are carbon – hydroxyl group bond (C – OH bond), oxygen – hydrogen bond (O – H bond) and the hydrogen attached to α-carbon. These bonds are involved in chemical reactions of alcohols.
- Dehydration of alcohols yields alkenes.
- Esters are important derivatives of alcohols formed by their reaction with carboxylic acids.
- Oxidation of primary & secondary alcohols forms aldehyde or carboxylic acid. However, tertiary alcohol does not undergo oxidation reaction.
Alcohols
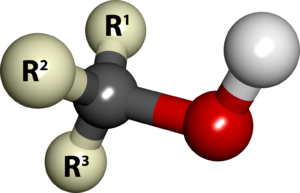
Alcohols are class of organic compounds containing hydroxyl group attached for carbon atom of alkyl chain. A generic formula is shown below. Here hydroxyl group (-OH) is attached directly to carbon atom of alkyl group ( R) which could be methyl, ethyl, propyl or even long chain alkyl group.

Lower carbon chain alcohols such as methyl alcohol (methanol) or ethyl alcohol (ethanol) are common lab solvents. Alcohols find several industrial applications. Fermentation of sugar gives ethyl alcohol that is further processed to formulate several alcoholic beverages.
The chemical properties of an alcohol are directly related to the presence of hydroxyl group. Hydroxyl group creates slight polarity in an alcohol molecule. There are three sites responsible for the chemical reactivity of alcohols. The carbon – hydroxyl group bond (C – OH bond), oxygen – hydrogen bond (O – H bond) or the hydrogen attached to α carbon (C – H). The carbon directly attached to hydroxyl group is called α-carbon.
Cleavage of C – OH bond
Dehydration: Formation of alkenes
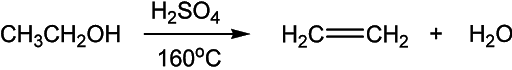
An alcohol is converted into an alkene by dehydration in the presence of an acid and heat. This is the elimination of water a molecule.
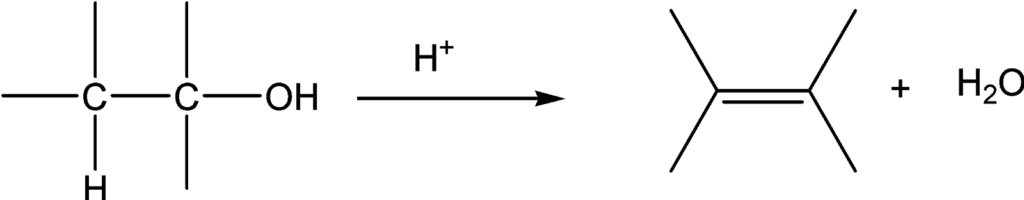
The reaction is catalyzed by the presence of an acid. Sulfuric acid or phosphoric acid is commonly used for this purpose. In the first step, alcohol is protonated and subsequently, the carbon-oxygen bond undergoes heterolysis and hydroxyl group leaves forming a carbocation. In the last step, carbocation loses proton to conjugate base of acid to form alkene.
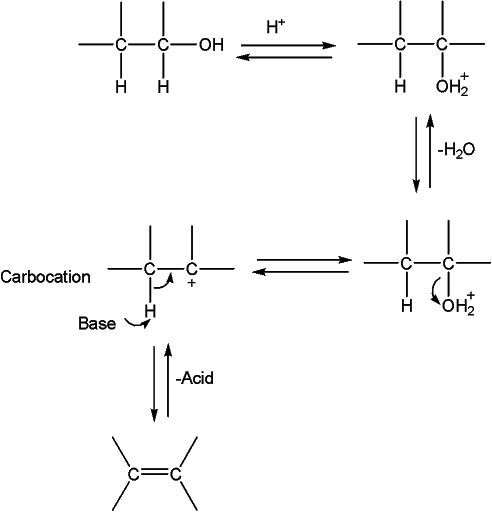
Different alkyl groups attached may influence the formation & stability of carbocation. As we know, tertiary carbocation is more stable than primary, that’s why tertiary alcohols show high reactivity compared to primary alcohols.
Order of reactivity tertiary > secondary > primary
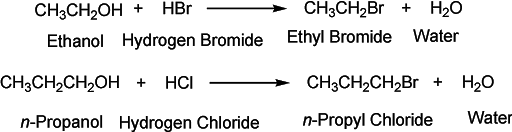
Reaction with hydrogen halides
The reaction of alcohols with hydrogen halides (HCl or HBr) forms alkyl halides. This is a substitution reaction where one nucleophile displaces the other nucleophile. The rate of reaction depends upon the nature of substrate alcohol whether it is a primary or secondary or tertiary alcohol.
The reaction mechanism involves the same steps as in the dehydration of alcohols. In the first step, the alcohol molecule is protonated which proceeds to form a carbocation. This carbocation then combines with an electron-rich halide anion to form an alkyl halide. The order of reactivity is the same again.
Tertiary alcohol > secondary > primary
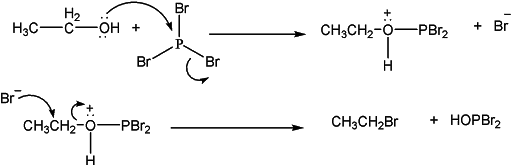
Reaction with PX3 & PX5
Primary and secondary alcohols react with phosphorus halides to give alkyl halide. For example, phosphorus tribromide is used to form bromoalkanes from alcohols. All three bromide atoms can be transferred to the alkyl group.

The reaction proceeds via the formation of a protonated inorganic ester of phosphoric acid. In the subsequent step, the other two bromide anions attack the alkyl group, and HOPBr2 leaves as a leaving group. The reaction continues until all PBr3 is fully consumed.
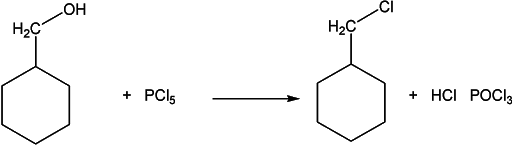
For the preparation of chloroalkanes, phosphorus pentachloride is used. The reaction mechanism is the same, except, now we have pentavalent phosphorus.
Reaction involving O – H bond cleavage
Acidity of Alcohols: Reactions with active metals
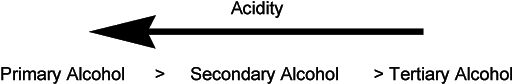
The basicity of alcohols is observed in the above examples where the hydroxyl group was protonated in the presence of strong acid. At the same time, alcohols can be acidic in nature as their pKa is close to water. Hydrogen is bonded to electronegative oxygen and thus O – H bond is polarized. This may facilitate the separation of proton and electronegative oxygen can accommodate the remaining electrons. However, important to remember, the acidity of alcohols greatly varies with their chemical structure. The alkyl group influences the acidic behavior. For example, primary alcohol is slightly more acidic than secondary alcohols which in turn are more acidic than tertiary alcohols.
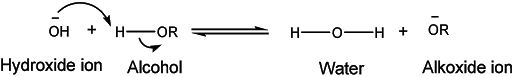
The acidic nature of alcohol is demonstrated by its reaction with metals to liberate hydrogen gas.
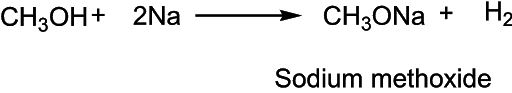
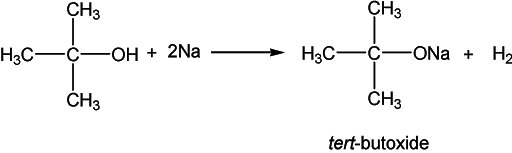
Esterification
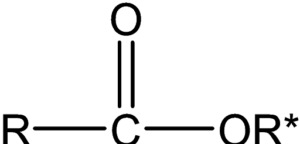
The reaction of alcohol with a carboxylic acid in the presence of mineral acid to form an ester is called esterification. It is an acid-catalyzed condensation reaction commonly known as Fischer esterification. A general formula for an ester is given as:
Adding alcohol to carboxylic acid, no reaction takes place. However, in the presence of a small amount of mineral acid, such as sulfuric acid or hydrochloric acid, the two reactants react and slowly form an ester. This esterification reaction is reversible and lies in equilibrium. The rate of forward reaction depends upon the nature and amounts of alcohols and carboxylic acid used. The rate of forward reaction (esterification) can be made favorable by having an excess of either alcohol or carboxylic acid. The other option to make esterification more favorable is by removing the product from the reaction mixture solution. Here, water can be removed by adding a cosolvent. The reaction in the backward direction (reverse reaction) is acid-catalyzed hydrolysis of the ester to give starting alcohol and carboxylic acid again.
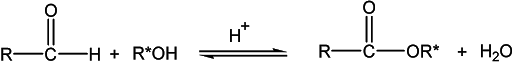
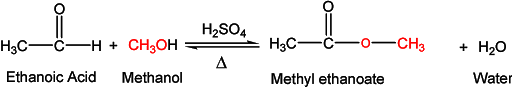
The reaction mechanism involves an interesting question. Whether oxygen in the ester linkage (C-O-C) comes from alcohol or carboxylic acid? The reaction was studied using isotopically labeled oxygen (18O) and results showed that alcohol oxygen is incorporated into ester.
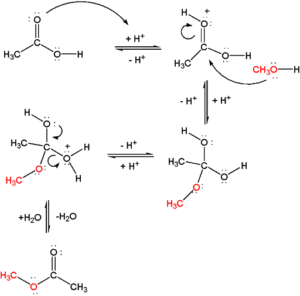
In this reaction, first, the acid is protonated that subsequently drives nucleophilic attack from methyl alcohol at carbonyl center. This forms a tetrahedral intermediate of nucleophilic addition. Later on, protonation at hydroxyl oxygen eliminates water and thus forms ester.
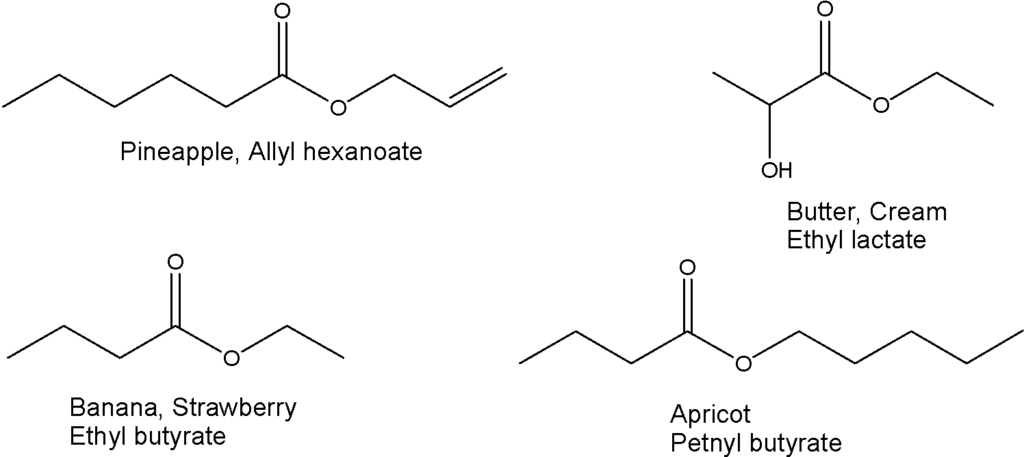
Esters are an important class of organic compounds as Mother Nature provides us a diverse range of esters present in fruits. They offer fruit-like odor and many occur naturally in the essential oils of plants. Esters are also commonly used in artificial flavorings and fragrances in drinks, beverages, and other confectionery products. Some examples are here:
Oxidation of alcohols
The oxidation of alcohol (adding more oxygen into alcohol molecule) gives carbonyl compounds. It can be aldehyde, ketone, or carboxylic acid. The product depends upon the nature of the alcohol and oxidizing agent used. The reaction involves the removal of one or more hydrogens (α – hydrogens) from the carbon of C – OH bond. For example, primary alcohols contain two α – hydrogens and can lose one of them to form aldehyde or it can lose both hydrogens to give carboxylic acid. Thus, the presence of α – hydrogen plays an important role.
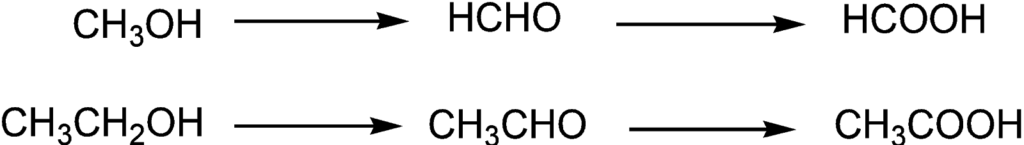
Primary alcohol such as methanol or ethanol gives formaldehyde and acetaldehyde which may undergo further oxidation to give formic acid and acetic acid respectively.
The oxidation product also depends upon the oxidizing agent used. Primary alcohols with potassium permanganate give carboxylic acid. However, the product or reaction can be stopped at aldehyde using a chromium (IV) oxidizing agent.
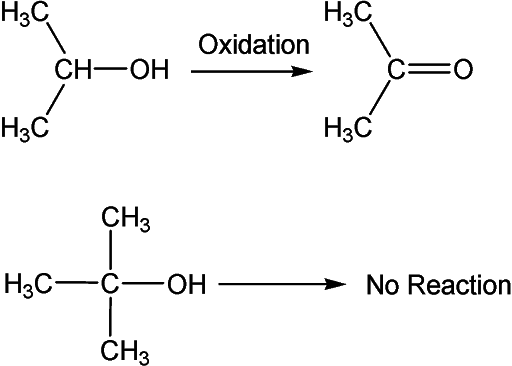
In the case of a secondary alcohol, it has only one α – hydrogen and hence gives only ketone (Acetone). However, tertiary alcohol such as iso-butanol does not contain any α – hydrogen and thus it does not undergo oxidation reaction.
Frequently Asked Questions
What are the three types of alcohol?
Alcohols are classified on the basis of position of the carbon atom to which the hydroxyl-OH group is attached. If the -OH group is attached to a carbon atom which is attached to only one alkyl group it is called primary alcohol. Similarly, if the hydroxyl group is attached to the carbon atom which is connected to two or three alkyl groups, the alcohol will be secondary or tertiary, respectively.
How alcohols can be converted into alkenes?
Alcohols can be converted into alkenes by dehydration of alcohols. This dehydration process takes place in the presence of acid and heat.
What is the order of reactivity of alcohols with hydrogen halides and the order of acidic strength of alcohols?
The order of reactivity of alcohols with hydrogen halides is “Tertiary alcohols > Secondary Alcohol > Primary Alcohol” Whereas, the order of acidic strength of alcohols is “Primary Alcohol > Secondary Alcohol > Tertiary Alcohol”
Which enzyme is responsible for alcohol metabolism in the body?
The most important enzymes involved in alcohol metabolism in the body are “alcohol dehydrogenase” and “aldehyde dehydrogenase”. They convert alcohol into Acetate which is eliminated from the body.
Books for further study
- Morrison, R. T., and R. N. Boyd. "Organic chemistry 5th edition." (1987).
- Cary, A, F. Organic Chemistry, 3rd edition, (1996).
- Volhardt K, P, C. Organic Chemistry, (1987).
- Smith M, B and March, J. March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th edition (2001).
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





