Table of Contents

Summary of Reaction Masses and Atom Economy
When a reaction occurs, we usually assume that the yield of the reaction is 100%. But this is not true in reality since the actual percent yield (or experimental yield) of a reaction is lower than the theoretical value.
There are several ways to determine the percentage of useful products as a result of a reaction.
The two most common terms that will be discussed throughout the article are the following:
- Percentage Yield – used to compare the number of products obtained to the number of expected products;
- Atom Economy – used to measure the number of reactants that become useful products.
Percentage Yield
As it was already mentioned above, the percentage yield is the value in % that describes the relationship between the theoretical and experimental yield of a particular reaction.
The theoretical yield of a reaction is defined as the maximum amount of product that can be obtained in a reaction.
The experimental yield of a reaction is defined as the experimentally determined amount of product that was actually obtained in a reaction.
To calculate the percentage yield, we use the simple and straightforward equation:
Percentage Yield = Experimental YieldTheoretical Yield x 100%
Experimentally, it is almost impossible to get the 100% yield in a reaction. But theoretically speaking, we can conclude the following:
- If the yield is 100%, it means that none of the products have been lost during the reaction, and the experimental yield is equal to the theoretical yield (expected yield).
- If the yield is 0%, it means that the expected product of a reaction was not obtained at all.
- If the yield is between 0 and 100%, it means that a certain amount of product has been obtained in a reaction, but the yield was less than it was theoretically expected.
There might be several reasons for not obtaining the expected yield in a reaction, including but not limited to, the following:
- Not all reactants were converted into the desired product;
- There was a loss during filtering or transferring the substance;
- The product was lost due to evaporation;
- Incorrect measurement of a product mass or volume.
NOTE:
Experimental and theoretical yield values must be plugged into the equation in the same units. For instance, in the scenario above, we used the mass of the desired product in grams for both experimental and theoretical yields. In case if one of the values is provided in moles and another one is in grams, you must convert either both of them to moles or grams.
Let’s consider several examples to understand the concept better.
Sample Problem:

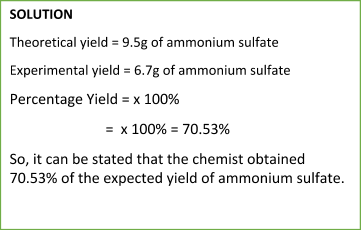
Sample Problem:


Sample Problem:

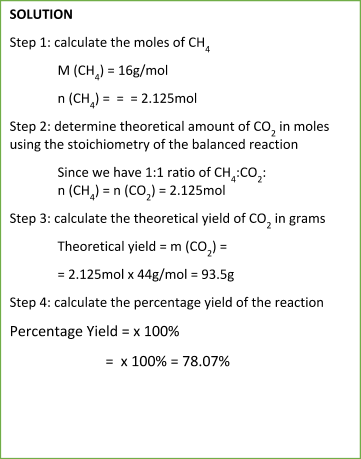
Read more about Factors Affecting the Rate of a Reaction
Atom Economy
As was already mentioned above, the atom economy is a term used to describe the relationship between the number of reactants and useful products.
There might be two types of reactions considering the atom economy:
- Efficient (more economic) reactions – atom economies are high; therefore, there are less undesired products obtained in a reaction. Reactant atoms are used to produce useful products;
- Inefficient (less economic) reactions – atom economies are low; therefore, there are more undesired products obtained in a reaction. Reactant atoms are wasted for unwanted products.
To calculate the atom economy, we can use the following equation:
Atom Economy = Mr of the desired productSum of Mr of all products x 100%
Atom economy is 100% for addition reactions since there are no additional products obtained in a reaction rather than the desired one:
A + B → AB
Let’s consider several examples to understand the concept better.
Sample Problem:

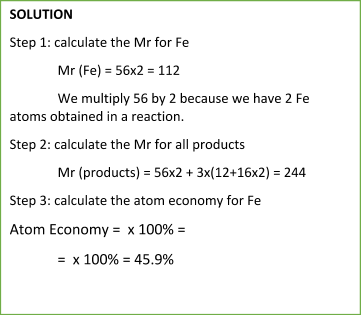
The equation for the atom economy can also be written in the following form:
Atom Economy = Mr of desired productSum of Mr of all reactants or products x 100%
It does not matter whether you use the sum of Mr for all reactants or the sum of Mr for all products.
According to the law of conservation of mass:
Mr (Reactants) = Mr (Products)
To make sure that this concept is valid, we can consider the same problem that we have discussed above. As we have written, the sum of Mr of all products in the reaction “Fe2O3 + 3CO → 2Fe + 3CO2” was 244.
Now, let’s calculate the sum of Mr of all reactants in the reaction. That would be the following: Mr(reactants) = 56x2 + 16x3 + 3x12 + 3x16 = 244
As you can see, the sum of Mr of all reactants is the same as the sum of Mr of all products in the reaction.
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.
Frequently Asked Questions
What is atomic mass?
Atomic mass is the sum of the total no. of protons and neutrons present in the nucleus of an atom. Unlike the atomic number, atomic mass of different isotopes of an element differs due to the difference in the no. of neutrons in different isotopes.
What is atom economy?
Atom economy deals with the effectiveness of conversion of all the atoms of reactants involved in a chemical reaction to the products. In other words, it tells us about the actual yield of a chemical reaction. The higher the atom economy, the higher the yield and vice versa.
What is the actual yield?
Actual yield or experimental yield is the amount of the product obtained after a chemical reaction takes place. Actual yield is always less than the theoretical yield due to different factors.
Is experimental yield equal to the theoretical yield?
No, experimental yield is always less than the theoretical yield due to different factors i.e., wastage of volatile reactants or products, improper handling, use of inappropriate/ineffective methods and improper environmental conditions etc.





