Table of Contents
Key Facts & Summary for Rates, Equilibrium and pH:
- Reaction rate is the number of reactant particles that react to form product particles per unit of time
- Reaction rate is influenced mainly by temperature, concentration, particle size and the presence of a catalyst
- Entropy is a measure of the disorder of a system
- Chemical equilibrium is the condition in which the forward and backward rates of a reversible reaction occur at the same rate
- A decrease in enthalpy (negative Hº value) favors a spontaneous reaction
Reaction rate
Reaction rate is the number of reactant particles that react to form product particles per unit of time.There are many factors that influence a reaction rate, but the 4 principal ones are the following:
Temperature - the higher the temperature, the faster is the reaction, as the particles move faster and they are more likely to interact with other reagents and they will have enough energy to overcome the energy barrier to form products.
A general rule is that for every 10 ºC rise in temperature the reaction rate doubles.
Concentration - the higher the concentration the higher the reaction rate. The more particles are present, the more likely they are going to collide with each other and react.
Particle size - the smaller the particle size, the greater the surface area. The increase in surface area makes it more likely that reactant particles will collide to react to form product particles.
Catalysts - catalysts reduce the activation energy needed for a reaction to occur and so increase the number of effective collisions. Catalysts speed up reaction rate but they are not directly involved in the chemical reaction.
Read more about factors affecting the rate of a reaction
The collision theory
The collision theory states that in order for reactants to react, and change into products, they must collide or come into contact with each other. A collision is effective if it has enough energy to overcome the energy barrier. This is called activation energy. A collision needs to happen with proper orientation in order to be effective: reactant particles must collide in the correct positions to break and reform bonds
Entropy
Entropy is a measure of the disorder of a system. The law of disorder states that things move spontaneously in the direction of maximum chaos or disorder. In general, solids have the least amount of entropy as their structure is well ordered and crystalline. Liquids have less order as the molecules can move more freely about, and gases have the most entropy. That said, we can conclude that the entropy of a substance will increase as it changes from a solid to a liquid to a gas.
Writing down chemical equations it can be seen that there is more entropy on the side that has a greater number of pieces. In the equation for photosynthesis, for example
6CO2 + 6O2 → C6H12O6 + 6O2
There is more entropy in the left side of the reactants (12 molecules) and less entropy in the products (7 molecules). Entropy is designed with the letter S, and an increase of it, therefore a positive value for Sº, favours a spontaneous reaction.
Enthalpy
Enthalpy (H) is a measure of the heat content of a substance at a given temperature and pressure. Standard enthalpy (Hº) is the enthalpy of a substance at 25ºC and 1 atm of pressure. The equation for the change in standard enthalpy is
ΔHº = Hº(products) - Hº(reactants)
A decrease in enthalpy (negative Hº value) favours a spontaneous reaction.
Chemical equilibrium
Reversible reactions are reactions in which the reactants change into the products and the products change into the reactants simultaneously.
![]()
Chemical equilibrium is the condition in which the forward and backward rates of a reversible reaction occur at the same rate. This condition is dependent on the reaction environment: any change in temperature or pressure may cause the reaction to shift its equilibrium position towards the reactants or products (towards the left or the right).
The equilibrium position of a reaction is determined by the relative amounts of reactants and products at equilibrium. If we add more oxygen to the above reaction the equilibrium will be shifted to the right. If we add more products, the equilibrium is shifted to the left. This is called also Le Châtelier's principle states which states: if a stress is applied to a system at equilibrium, the system will shift its equilibrium position to relieve the stress.
The equilibrium constant Keq for a reaction at equilibrium is the ratio of the product concentrations to reactant concentrations, each raised to the power of the number of moles there are in the balanced equation. For example the above reaction
![]()
would have the equilibrium expression of:
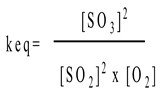
The factors influencing the equilibrium of a reaction :
There are many factors that can influence the equilibrium of a reaction. These factors are :
- Changes in concentration: changing the concentration of reactants or products by the addition or removal of reactants or products will shift the equilibrium position. As said above, If we add more oxygen to the above reaction the equilibrium will be shifted to the right. If we add more products, the equilibrium is shifted to the left. Chemists can use this to increase the amount of product formed by removing the product of a reversible reaction.
- Changes in temperature: changing the temperature of a reaction at equilibrium will shift the equilibrium position to undo the stress of the temperature change. You can treat temperature just like a reactant or product.
- Changes in pressure: changing the pressure on a system at equilibrium will change the equilibrium position of reversible reactions with unequal numbers of moles of gaseous reactants and products. It will not affect non-gaseous reactants or products. This is really the same as changing the concentrations of the gaseous reactants or products. Increasing the pressure on a gas compresses it increases the density or concentration of the gas.
pH
[H+] is shorthand notation for the concentration of H+ ions in water - which is what makes acids acidic. In reality, H+ is present in water in the form of H3O+ ions. For simplicity, we don't usually bother to distinguish between them.
Concentrations of H+ ions are very small even in fairly concentrated weak acids and especially in strong bases.
For instance, a weak-ish alkali may have [H+] = 0.000000055 - which is an awkward number. Taking the Log makes it somewhat less awkward and easier to understand and work with:
Log10(0.000055) = -7.26
And since everyone is happier with positive rather than negative numbers we could ignore the negative sign and simply use 7.26.
Therefore, the definition of pH is: pH = - Log10([H+])
Frequently Asked Questions
What are the different factors affecting the rate of a chemical reaction?
Several factors affect the rate of a chemical reaction, such as the concentration of the substrate, nature of products, temperature, and presence of a catalyst.
What is chemical equilibrium?
Chemical equilibrium is when the rate of forward chemical reaction becomes equal to the rate of reverse reaction so that the reaction mixture becomes constant.
How do you define pH?
pH is the -ve log of H+ ion concentration in a solution.
pH= - log [H+]
Which reactions at equilibrium are affected by a change in pressure?
Pressure changes at equilibrium affect those reactions having an unequal number of gaseous reactants and products at the equilibrium stage.
Sources
https://en.wikipedia.org/wiki/Reaction_rate





