Table of Contents
In every chemical reaction, the concentration of reactants (starting compounds) and those of the products (resulting compounds) change as time passes by. The measure of the change in the concentration of the reactants or products per time unit is referred to as the reaction rate for a particular chemical reaction. The reaction rate is defined as the speed with which the concentration of products or reactants changes. Keep reading to learn more about factors affecting the rate of a reaction...
Obviously, there are various factors affecting the rate of a chemical reaction, and the study of these factors is considered to be chemical kinetics.
Until we continue with the specific factors that influence the speed of a reaction, let’s define some of the general terms and concepts related to the reaction rate.
If we consider any chemical reaction in which reactants are abbreviated as “A” and “B” and the product of a reaction is “AB”:
A + B --> AB
We will notice that as time goes by, the concentrations of reactants “A” and “B” decrease and that of product “AB” increases.
This relationship between the time and the molar concentration of the reactants and products is represented on the chart attached below:
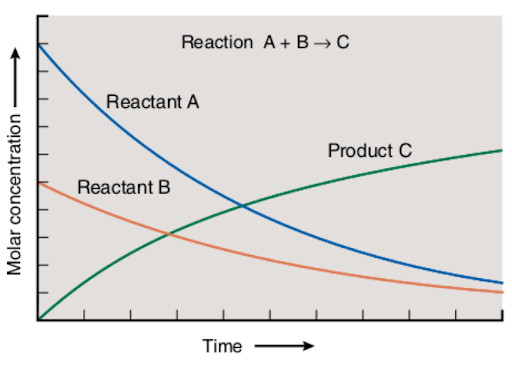
The decrease in the molar concentration of reactants and the increase in the molar concentration of the product is a simultaneous process. As the reaction proceeds, more reactants are transformed into products, causing the changes in the molar concentrations of starting and resulting components of a reaction.
The rate of a reaction can be calculated using the following two formulas:
Rate = Moles of product formed time elapsed
Rate = Moles of reactants transformed time elapsed
Let’s consider the following sample problems to make sure that the equations provided above are correct and give the same value for the rate of a reaction.
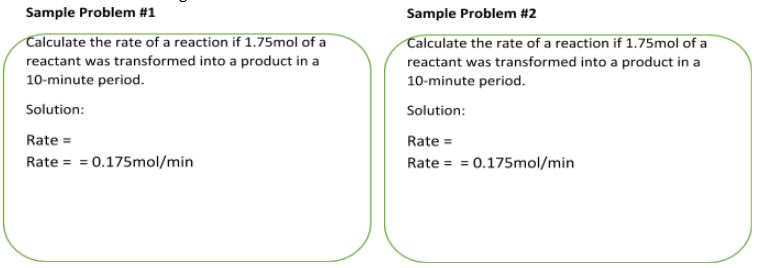
As you can see, the rate of a given reaction has the same positive value in both cases. So, you can calculate the reaction speed using either of the two equations.
Another concept that we should also consider is the collisions between the reactant molecules. Collisions are required for every reaction to take place. Collisions between reacting species that result in a reaction are referred to as reactive collisions (successful, effective collisions).
Altering the rate of collisions results in a change in the speed of a given reaction.
The major factors affecting the collision rate (and hence of a reaction) are the following:
- Concentration of reactants
- Temperature
- Presence of a catalyst
- Spatial orientation of reactant molecules
- Phase of reactants
- Surface area of reactants
Concentration of Reactants
The concentration of reactant molecules has an impact on the reaction rate since the increase in the concentration of particles leads to more collisions between the molecules. The rapid collision of particles makes the reaction occur more often. Therefore, the rate of reaction also increases with the increase of the reactant concentrations.
To better understand the concept, check the image attached below:
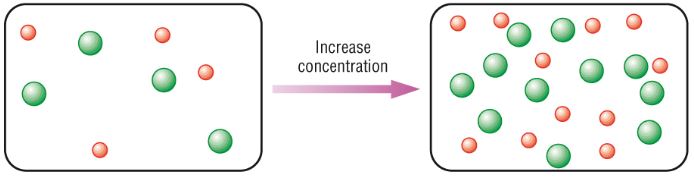
You can see, have two containers with reactant molecules. In the second container, we have increased the concentration of reactant molecules. It is obvious that there will be more collisions between the reactant molecules in the case of the second container.
In the case of gaseous samples, the increase in pressure also increases the concentration of the molecules, meaning that the reaction rate is also increased.
Consequently, we can conclude that if we increase the concentration of reactants, we increase the number of collisions; Thus, the reaction rate increases.
Temperature
As you may already know, reactant molecules need some energy for the reaction to take place. This minimum energy required for the molecules to react with each other is referred to as the activation energy.
The kinetic energy of a gas is directly proportional to temperature; therefore, increasing the temperature also increases the energy which means that the molecules are more likely to collide with each other with sufficient activation energy for the reaction to proceed.
As a result, if we increase the temperature of a system, we can increase the probability of the molecules moving and colliding with the necessary energy.
Let’s consider the following diagram (Maxwell-Boltzmann Distribution which will be discussed later in the article) to get a better idea of the relationship between the temperature and activation energy of reactant molecules:
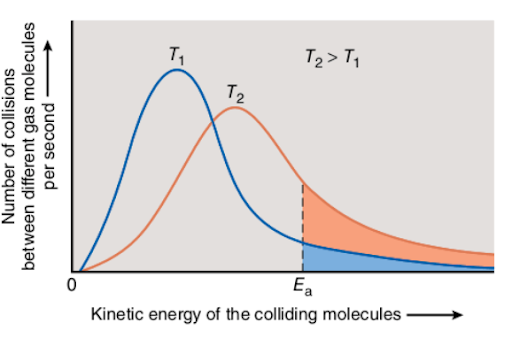
The diagram represents the number of collisions between the reactant molecules as a function of their corresponding kinetic energies at two different temperature values.
T1 is the initial temperature of the system, while T2 corresponds to the increased temperature. Therefore, T2 > T1.
Ea – the activation energy - the minimum energy needed for a reaction to occur.
Since the reactant molecules typically collide more rapidly in hot systems, the increase in temperature causes the increase in kinetic energy of the colliding molecules, as it is shown in the diagram above. As a result, the reaction occurs much faster than in the case of cold systems.
Presence of a Catalyst
Another important factor that significantly alters the rate of a chemical reaction in the presence of a catalyst. A catalyst is a compound that is used to increase the speed of a reaction but remains unchanged after the reaction has been completed.
There are two types of catalysts, positive and negative. Positive catalysts decrease the activation energy to increase the reaction rate, while negative catalysts (also referred to as inhibitors) increase the activation energy to decrease the speed of a reaction.
Positive catalysts are typically indicated simply as catalysts.
Catalysts play an essential role in our organisms in the regulation of different processes. Catalysts that are present in living organisms are called enzymes.
Some of the catalysts are so important that without them, the reactions would never occur.
Generally, catalysts cause the reaction to take place through different routes that have lower activation energy. Therefore, it is easier for the reactant molecules to collide, which leads to an increased reaction rate.
The following diagram shows the reaction progress of uncatalyzed and catalyzed reactions:
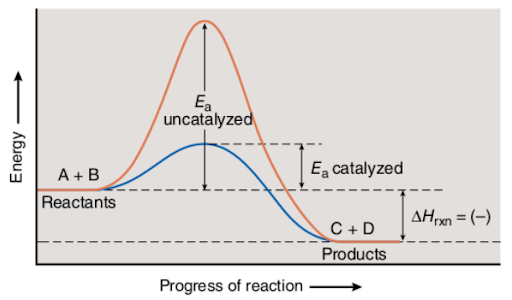
The red curve corresponds to the uncatalyzed reaction, while the blue curve is the one for the catalyzed reaction. Evidently, the activation energy that is also shown in the diagram is less in the case of the catalyzed reaction.
Note that even though a catalyst decreases the activation energy needed for the reactant molecules to cause reactive collisions, the energies (usually in the form of heat) of reactants (A and B) and products (C and D) in both, endothermic and exothermic reactions are unaltered.
We should note that the presence of a catalyst does not change the distribution of the molecular energies.
Consequently, we can state that a catalyst lowers the activation energy of a reaction through a new pathway without changing the result of a reaction. The only thing that is changed is the rate at which the reactant molecules collide with each other, hence the speed of a reaction.
Spatial Orientation of Reactant Molecules
According to the collision theory, reactant molecules must collide with sufficient energy and proper orientation in space for a reaction to take place. Since we already discussed the importance of activation energy, let’s continue with the spatial orientation of reactant molecules.
Even if two reactant molecules have enough activation energy to collide, we cannot say that the reaction will occur. Instead, they must also have correct spatial orientation for the reaction to take place. This guarantees the fact that designated atoms line up with each other and the bonds break and reform in the proper manner.
Since molecules in the liquid or gas phase are in constant and random motion, the probability of two molecules colliding in the proper orientation is quite high. In contrast to this case, orientation is essential for molecules that are more complex and larger in size. In such cases, the number of reactive collisions leading to the reaction is fewer; Thus, the rate of a reaction is also affected.
Phase of Reactants
Another factor affecting the rate of a reaction is the phase of reactants. If the reactants are in the same liquid phase, the particles collide more rapidly with each other than in the case of differing liquids (liquids that do not mix with each other) or solid reactants. If the reactants are in the same phases, the reaction is stated to be homogenous. On the contrary, if the reactant molecules are in two different phases, the reaction is referred to as heterogeneous.
The number of collisions in homogenous reactions is more than in the case of heterogeneous reactions (in some cases which will be discussed later in the article); Therefore, the rate of reaction of a homogenous reaction is also higher.
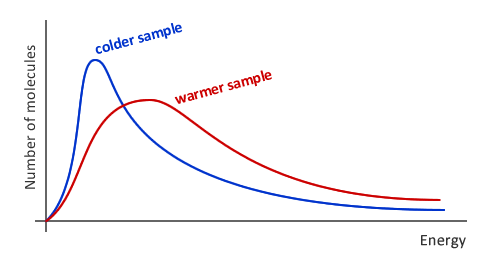
In case if the reactant molecules are in the gaseous phase, we can use the Maxwell-Boltzmann Distribution to define the relationship between the number of particles and corresponding energies at a constant temperature. We have already discussed the Maxwell-Boltzmann curve in the section “Temperature” and what we have concluded is that the increase in temperature resulted in a shift of a curve, meaning that the particles collide with greater energy in hot systems; Therefore, the reaction rate is increased.
Looking at the Maxwell-Boltzmann distribution curve provided above, we should note that the area under the curve represents the total number of particles in a system. The blue curve is the one with the lower temperature, while the red curve is the one with the higher temperature. The change in the temperature does not alter the area under the curve, meaning that the total number of particles remains the same. Although, the particles in a sample have more kinetic energy and move faster. Due to the fast movement, the probability of reactive collisions increases, which significantly alters the reaction rate (reaction rate increases as well).
Surface Area of Reactants
As it was mentioned above, the rate of a reaction is also dependent on the phase of the reacting substances. In the case of heterogeneous reactions (when reactants are in different phases), one of the reactants might be in the solid phase (or more condensed phase than the other). In such cases, the rate of a reaction is affected by the surface area of a solid or more condensed phase reactant. The larger the surface area of such reactant, the higher the rate of a given reaction.
This happens due to the fact that the larger surface area of a compound leads to more collisions between the two reactants with different phases. Therefore, an increase in the number of collisions also increases the reaction speed.
Summary
The major concepts and terms mentioned throughout the article are summarized in the table below:
| Chemical Kinetics | The study of the factors affecting the rate of a reaction. |
| Rate of a Reaction | The measure of a change in concentration of the reactants and products per time unit. Rate = Moles of product formed time elapsed Rate = Moles of reactants transformed time elapsed |
| Reactive Collision | A collision of molecules resulting in a reaction. Also referred to as an effective, or successful collision. |
| Activation Energy | The minimum energy required for the reaction to occur. |
| Factors Affecting the Rate of a Reaction | Concentration of reactants - Increase in concentration ???? increase in reaction rate temperature - increase in temperature ???? increase in reaction ratePresence of a catalyst - increases reaction rateSpatial orientation of reactant molecules - proper spatial orientation is crucial for the reaction to occur has of reactants - reaction rate increases if the reactants are in the same phase surface area of reactants - surface area of solid or more condensed phase reactants increases the reaction rate |
| Maxwell-Boltzmann Distribution Curve | Shows the relationship between the particles and their corresponding energy values. The area under the Maxwell-Boltzmann distribution curve represents the total number of particles in a sample. The increase in temperature does not change the area under the curve. |
Frequently Asked Questions
What are the different factors affecting the rate of a reaction?
The rate of reaction depends upon several factors such as concentration of reactants, presence of a catalyst, temperature, the spatial orientation of reacting molecules, the surface area of reactants, nature of products etc.
How to calculate the rate of a reaction?
The following mathematical formulas are used to calculate the rate of reaction:
Rate of reaction= Moles of product formed/Time
Rate of reaction= Moles of reactants transformed/ time
How the concentration of reactants affects the rate of reaction?
The rate of reaction depends upon the collision frequency of the reacting molecules. As the reacting molecules are increased, the collision frequency increases, so the rate of reaction increases.
How temperature affects the reaction rate?
An increase in temperature boosts the kinetic energy of reacting molecules and their collisions. As a result, the reaction rate increases.
References:
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.






