Table of Contents
Summary
- Ethanol is an important organic compound with various lab and industrial applications.
- At industrial level, the production of ethanol occurs through the hydration of ethene.
- Ethanol is also prepared by fermentation of starch.
- Due to recent usage of ethanol as fuel. Its demand is increasing globally.
- Both ethene and fermentation method compete against each other for cost and environmental sustainability.
Ethanol

Ethanol or ethyl alcohol is an important organic compound. In general, it is also referred to as alcohol spirit, spirit of wine, grain alcohol, absolute alcohol, and ethyl hydrate. Its chemical formula is C2H5OH and has a molecular mass of 46.07g/mol. It is a colourless liquid with a characteristic odour. It is a psychoactive substance and is the main component of alcoholic beverages.
Ethanol is used as a solvent in the lab and has many industrial uses. Recently ethanol has been in the news headlines highlighting the economic value of ethanol usage. This has attracted new energies in the industrial production of ethanol. It has been used as car fuel as an alternative to gasoline. Certain countries such as Brazil have been using it as light vehicle fuel. In the USA, ethanol is either used alone or mixed with gasoline.
Production of ethanol
Ethanol can be produced synthetically using ethene or can also be made by fermentation of sugar using microorganisms. The cost of either procedure depends upon the cost of raw material. Ethene is obtained from petroleum while corn is the main source of sugar for the fermentation process. Petroleum ethene being cheaper offers cost-benefit for synthetic industrial-scale production of ethanol.
From ethene to manufacture ethanol
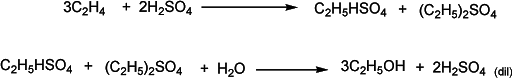
Ethanol is made by hydration of ethene (CH2=CH2). This is the easiest and most cost-effective method to manufacture ethanol. Ethene is a product of the petroleum industry and thus is an easily available raw material. The reaction is carried out in the presence of phosphoric acid (H3PO4) used as a catalyst. Phosphoric acid is coated over silicone dioxide. Ethene is mixed with steam in a fixed molar ratio of ethene/water (1:0.60). The mixture is heated to 300 oC and the gases react over the catalyst to form ethanol. The gaseous mixture of unreacted ethene and product ethanol is cooled down which liquefies ethanol. The product is separated and unreacted ethene is recycled back into the reaction chamber. The productivity per cycle (or per pass) is 5.0 – 25.0% depending upon the activity of the catalyst. High yield is achieved by recycling ethene and by removing ethanol from the reaction system. The chemical equation can be written as:

Another method to convert ethene into ethanol is via ethyl sulfate. Ethene reacts with sulfuric acid to make ethyl sulfate which on hydrolysis gives ethanol. A common by-product is an ether that is separated.
Fermentation procedure for ethanol production
Fermentation is a biochemical process carried out by bacteria, yeast, or other organisms converting sugar such as glucose and fructose into cellular energy and producing ethanol and carbon dioxide as by-products. The whole process also releases heat energy. Alcoholic fermentation is one the oldest and most important industrial processes known to mankind. Humans learned this process thousands of years ago. Traditionally fermentation has been used to make alcoholic beverages, but today it adds a lot of economic value to the global economy. As mentioned earlier, ethanol today is used as a source of energy for vehicles. Thus its demand has increased in recent years. Other than ethanol, scientists have developed various other techniques to make other chemicals of pharmaceutical and biological importance using fermentation. Today, vitamins, various antibiotics, enzymes, and other industrial chemicals are also made by fermentation at an industrial scale.
To make alcoholic beverages, traditionally, we have been using cereals, barley, and grapes. Basically, we need a source of starch that can be cereals, corn, or even some suitable wood stocks. However, with its production now at a large industrial scale, using barely and grapes is not economically viable. A new feedstock in the USA market has been corn. The government agencies have been proposing to use wood stock or corn to make ethanol. Though even this process is not cost-effective compared to ethene-based industrial synthesis, still, government subsidies and incentives are encouraging industrialists to use the fermentation process as a green and sustainable operation for ethanol production.
Fermentation
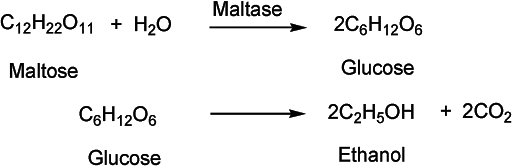
Starch is a complex carbohydrate and we need to break this complex structure into smaller sugar units such as maltose, fructose, and glucose for ethanol production. The whole process for corn fermentation involves series of steps. It starts with cleaning and washing of raw corn to remove dirt and other impurities. The raw material is crushed and heated in warm water to extract its starch content. The material is transferred to a fermentation tank where it is mixed with yeast. Yeast as we know is the source of enzymes for this biochemical process. Enzymes convert the complex starch molecules into maltose that in further biochemical steps converted into simple glucose units. This chemical reaction looks simple written in a chemical equation, however, the fact is, it is a complex chemical reaction involving various intermediate steps. The material is kept at a warm temperature (30-35 oC) in airtight environment. Oxygen interferes with this process and may oxidise ethanol to acetic acid (vinegar). Yeast microorganisms start eating sugar and producing ethanol. Their activity continues until the system gets to 8-12% ethanol level. Above this, their activity diminishes and reaction comes to a halt.
Distillation
The fermented material, known as mash, is further processed using one single step distillation to separate liquid from solid. The liquid is impure and contains only 8-12% ethanol. The solid is in a slurry form and contains a significant level of organic mass. This can be used additive for animal feed or is burned as an energy source.
The distillation of liquid increases the purity level of ethanol and the final product is 96% ethanol. This is used for various lab-scale or industrial applications. It still contains some water and hence is not suitable for the gasoline mixture.
In the end, 25kg of corn gives approximately 9 to 10 liters of ethanol.
Denatured alcohol
Alcoholic beverages are a major and important industry that contributes significantly to tax revenue. In certain countries, these beverages are heavily taxed. As ethanol has many other uses, to reduce the tax burden over ethanol, it is made unfit for drinking purposes. This is called denatured alcohol. Ethanol is mixed with some bittering or toxins such as methanol, denatonium benzoate, or pyridine to denature alcohol.
Absolute Alcohol
Complete removal of water gives us absolute or anhydrous ethanol. It is almost 99.6% ethanol with a trace amount of other liquid such as benzene added to give an azeotropic mixture. Dehydration of ethanol can also be carried out using molecular sieves. Absolute alcohol is used as solvent where water is undesired or water can react with other chemicals. In spectroscopy, water strongly absorbs in the ultraviolet region and hence absolute ethanol is a preferred choice as a solvent. Also, this purity grade is used as a fuel mixed with gasoline. Ethanol-containing water will not mix with gasoline and thus water must be removed completely.
Frequently Asked Questions
What are the two methods of ethanol production?
Ethanol can be produced by the chemical transformation of ethene or by fermentation of starch.
What is the cost-effective way of ethanol production?
Ethanol is produced from ethene or starch. Ethene obtained from petroleum is the cheaper way for industrial ethanol production.
How is ethanol prepared from ethene?
Ethanol is prepared from ethene by hydration in the presence of phosphoric acid acting as a catalyst. Ethene is mixed with heated steam in a fixed molar ratio. They react at 300-degree celsius to produce ethanol.
What is absolute alcohol?
Absolute alcohol is the anhydrous alcohol obtained after removing water completely from ethanol. It contains about 99.6% ethanol.
Books for further study
- Austin GT. Shreve’s chemical process industries: 5th Ed. 2012. McGraw Hill.
- Ethanol: in Ullmann's encyclopedia of industrial chemistry. 7th Ed. 2016.
- Ali MF, Ali BME, speight JG. Handbook of industrial chemistry. 2005. McGraw Hill.
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





