Table of Contents
Overview of Acids and Bases
Acids and bases are crucial to almost all the chemistry that we know - whether industrially relevant or of academic interest. Many reactions require an acidic or a basic medium to reach the desired products. So, it's essential to know what an acid or base is.
The chemical behavior of many compounds can be explained with their acidic or basic properties. According to Bronsted-Lowry definition, an acid is any compound that can transfer proton to other compounds (proton donor) and a base is any compound that can accept proton (proton acceptor). Acids are usually compounds that contain hydrogen bonded to electronegative element with polar covalent bond, and bases usually have lone pair of electrons. Example of an acid-base reaction:
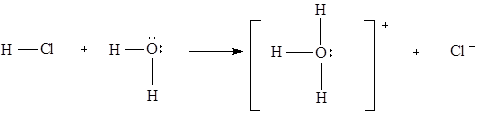
Polar HCl is acting as a proton donor, and the molecule of water, due to the two lone pairs of electrons in the oxygen, is acting as proton acceptor.
When the base accepts proton, the product is called conjugate acid of the primary base, and the product of acid that lost proton is called conjugate base of the primary acid.
There are compounds that can behave as an acid and a base depending on the circumstances, ex. water (amphoteric species).
As a consequence, the molecules of water can react with one another (autoionization of water) even in water that is not pure and produce equilibrium:
![]()
The constant of equilibrium is:
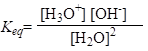
There are small numbers of H3O+ (H+) and OH- ions produced, so practically the concentration of water is approximated as being equal to unity, and this allows to:
![]()
Kw is known as ionic product of water. Its value at temperature of 25ºC and 0 ionic strength is constant and equal to 1.0 x 10-14 mol2dm-6.
The concentrations of OH- and H3O+ are equal in pure water (neutral solution). As a consequence, their concentration can be calculated:
![]()
Acidic solutions are those that contain more H+ than OH- ions. The opposite ones are basic.
The most commonly used unit for measurement of acidity or alkalinity of solutions is pH.
It is defined as negative of the logarithm to base 10 of the molar concentration, measured in units of moles per liter, of hydrogen ions:
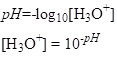
As a result, an increase of pH in one unit, corresponds to ten-fold decrease in c(H+), and opposite.
Neutral solutions (pure water) have pH=7. Acidic solutions have pH<7, and basic solutions pH>7.
Since the temperature can also affect the equilibrium, at higher temperatures pH of pure water can be less than 7.
Acids (and bases) can be differed by the ability to give (accept protons). There are strong acids that dissociate completely in water, and weak acids that dissociate incompletely.
Since strong acids dissociate completely in water, c(H+) is the same as the concentration of the acid, and pH is calculated using c(acid). (H+ ions that come from autoionization of water can be ignored).
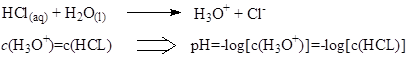
Dissociation of weak acids in water is incomplete and it is represented as an equilibrium.
![]()
The equilibrium constant is:
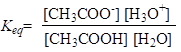
The concentration of water has huge value and it is considered to be constant:
![]()
This is known as acidity constant. Its value can be used to determine the strength of the acid. Reactions of dissociation of strong acids have equilibrium shifted to the right and the acidity constant has larger value, and this is opposite for weak acids. For practical purposes, pKa is also used:
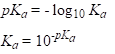
c(H+) is the same with c(A-), if the H+ ions present due to the autoionization of water are ignored, so for calculating pH:
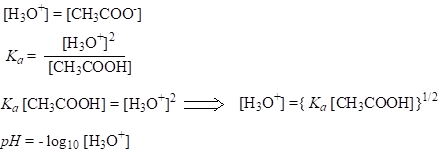
For weak acids, pH doesn’t increase for one unit, if the acid is diluted ten-fold. In other words, the amount of water affects the dissociation of weak acids.
The acidity of a particular solution depends on the concentration of the solution (dilute or concentrated solutions), but also on the strength of the acid (weak or strong).
When solutions are very dilute, c(H+) due to autoionization of water is significant and it can’t be ignored.
Acids that can donate more than one proton are known as polybasic acids:
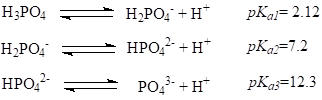
Bases can also be strong and week. pH calculation for basic solutions (strong base):
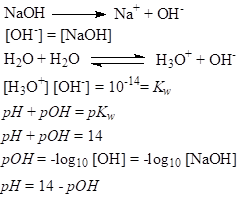
BUFFER SOLUTIONS
A buffer solution is a mixture of a weak acid and its conjugate base, or vice versa, and it is used to resist changes in pH when a small amount of an acid or a base is added.
Working principle:
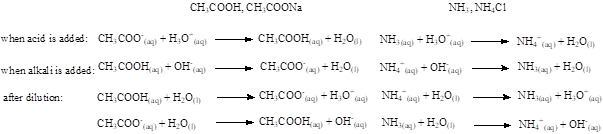
Species that can behave as weak acids and bases at the same time can also be used as buffers, for example aminoacids.
For pH calculation of buffer solutions, Henderson-Hasselbalch equation is used:
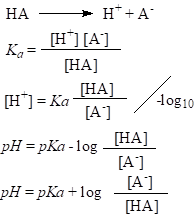
For example:
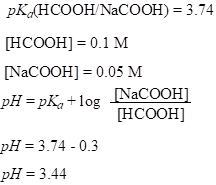
Buffer solutions can be effective in the range of pKa±1 because outside this range c(acid or base) is too small to resist changes in pH. Buffers are most effective when c(acid)=c(base), then pH=pKa.
There are many applications of buffers. They play vital role in regulating blood’s pH (cabronate and bicarbonate, phosphate, proteins, aminoacids).
TITRATIONS
Titration is a method used to determine the unknown concentration of an analyte, based on the reaction of neutralisation (reaction between acid and base). It requires usage of titrant (standard solution with known concentration and volume in a burette) and analyte (titrand) with known volume and unknown concentration in an erlenmayer flasc. Titration occurs until the reaction reaches neutralization and the equivalence point is determinated with usage of indicator (weak bases or acids that change treir colour when accept or donate protons). The pH range of the colour change of the indicator must correspond with the pH range of the equivalence point. The volume of reacted titrant is measured and used to determine the unknown concentration.
Strong acid–strong alkali pH calculation:
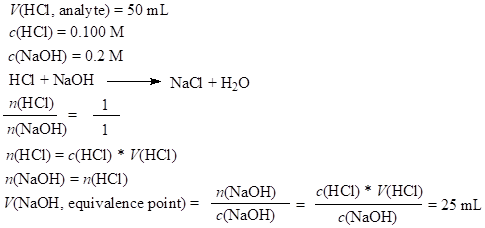
Before the equivalence point, ex. V(NaOH) = 15 mL
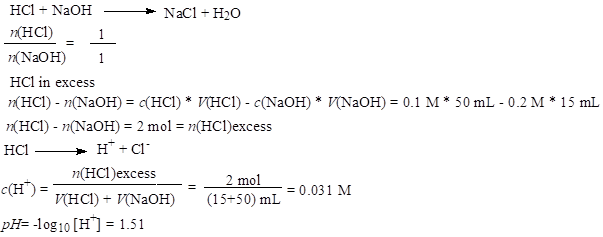
In the equivalence point, pH=7, and after the equivalence point, pH is calculated by the excess of OH- ions.
Titration curve is a plot of pH versus the volume of added titrant.
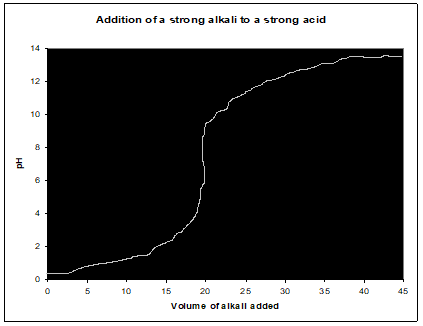
Weak acid–strong alkali (CH3COOH, NaOH)
- begining of titration-pH of weak acid (weak acid in excess)
- before the equivalence point-pH of buffer
- in the equivalence point–pH of salt that is formed
- after the equivalence point–by the excess of OH- ions
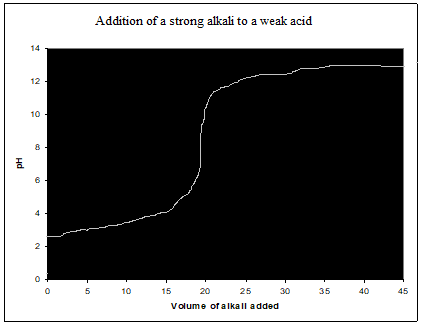
Strong acid-weak alkali titration curve (HCl, NH3)
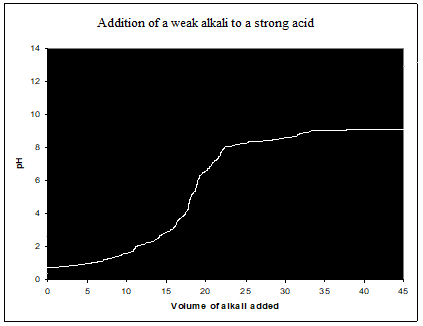
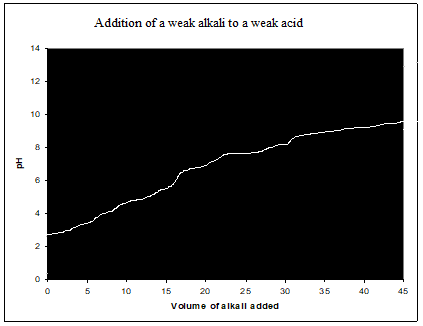
Weak acid-weak alkali (CH3COOH, NH3)
Frequently Asked Questions
What is a base?
According to Bronsted-Lowry acid-base classification, “a base is a compound which can accept a proton from a donor”. For example; H2O, NH3, NaOH etc.
What is an amphoteric species?
A compound which can act both as an acid and a base according to Bronsted-Lowry acid-base classification, depending upon the conditions, is called an amphoteric substance. Water is the best example of such a species.
What is the principle of buffer action?
Buffer solution works on Le Chatelier’s principle that if an equilibrium is disturbed by any change, the system will try to re-establish the equilibrium as much as possible”. When an acid is added to a buffer solution, it donates the protons resulting in pH drop, these protons will be accepted by the base component of the buffer and pH will be maintained.
What is meant by titration?
Titration is a technique commonly used in chemical analysis. It is used to measure the concentration of an analyte based on the reaction between the analyte and titrant solution. Acis base titration is a common example.
References:
- Khan Academy: https://www.khanacademy.org/science/chemistry/acids-and-bases-topic
- Chemistry Libretext: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)





