Table of Contents
Le Chatelier’s Principle is used for qualitative predictions of how a chemical system will respond to an alteration of its equilibrium conditions by means of change in temperature, pressure, or concentration of reactants and products.
The concept of this principle is closely related to the idea of chemical equilibria and equilibrium constants. Therefore, you must have particular knowledge and understanding of these topics.
This article will briefly overview the major terms needed to better understand Le Chatelier’s Principle.

Starting with the reaction types, they might either be reversible or irreversible. Reversible reactions are the ones that proceed simultaneously in both forward and reverse directions. On the contrary, irreversible reactions only move in one direction, towards the products.
Reversible reactions are characterized by a chemical equilibrium, which is the state when the concentration of the reactants and products remains constant.
The term dynamic equilibrium refers to the state in which the changes are no longer observable, but the system is in constant motion. Reactants are continuously transformed into products and vice versa.
Obviously, any disturbance in the equilibrium conditions of a given reaction will impact the particular system. Le Chatelier’s Principle describes the responses of a system to an alteration of any variable impacting the equilibrium state.
According to Le Chatelier’s principle, any physical or chemical system in an equilibrium that faces a disturbance will adjust the conditions to restore the equilibrium state.
A typical example of a physical system that restores the equilibrium value by adjusting the conditions is the liquid-vapor system. Let’s consider that the volume of the gas phase was decreased by increasing the pressure (at a fixed temperature). Some of the gaseous water was condensed back to its liquid phase to reduce the increased pressure and restore it to its initial equilibrium state. If the volume of a gas-phase was increased through decreasing the pressure, the liquid water would vaporize to its gaseous phase to increase the vapor pressure and return to its equilibrium state.
Similarly, any chemical system that is in equilibrium can also do the same if it is temporarily displaced from equilibrium. But the changes that the chemical system undergoes is somewhat different from the ones in the case of the physical system. These changes implemented by a chemical system include the alteration of amounts of reactants relative to products. Depending on the specific change in a system, the mass of a balanced chemical equation might shift from left to right or vice versa.
Dynamic equilibrium can only happen in a closed system at a constant temperature where there are no components added or removed from a system. If the closed equilibrium is opened, equilibrium conditions are affected by the changes in different variables. As we mentioned earlier, the system readjusts the conditions to reach the initial state of equilibrium again.
Read more about Equilibrium Constants
The major factors that influence the equilibrium are the following:
- Changes in the concentrations of reactants or products
- Changes in the pressure of reactants or products in the gas phase
- Changes in the temperature
Changes in the Concentration of Reactants or Products
The first factor that will be discussed in the article is the change in the concentration of any component of a chemical reaction at an equilibrium state.
What would happen if the concentration of either reactants or products was increased?
To answer the question, we should determine what would happen to the rate of a reaction.
So, if we increase the concentration of reactants, the rate of the forward reaction would increase. Therefore, more products will be formed, and the equilibrium will shift to the right. In such case, increasing the concentration of reactants favours the forward reaction.
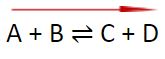
On the other hand, if we increase the concentration of products, the rate of the reverse reaction will increase. Therefore, more products will be transformed back into reactants, and the equilibrium will shift to the left. In contrast to the first case, increasing the concentration of products favours the reverse reaction.
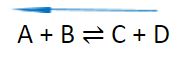
Changes in the Pressure of Reactants or Products in Gas Phase
In case if the reactants or products are in the gas phase, equilibrium is dependent upon the changes in the pressure.
To predict which side the equilibrium shifts as a result of an alteration of the pressure of reactants or products, you should consider the number of gas molecules on each side of the balanced chemical equation.
To do so, you just consider the balancing coefficients of reactants and products.
Let’s consider the following general chemical equation to better understand the concept:
As you can see, we have 4 molecules on the left and 2 molecules on the right.
If we increase the pressure, equilibrium will shift to the side with fewer molecules present. Since there are 4 molecules on the left and 2 molecules on the right, increasing the pressure will shift the equilibrium to the right.
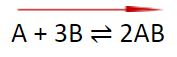
If we decrease the pressure, equilibrium will shift to the side with more molecules present. Since there are 4 molecules on the left and 2 molecules on the right, decreasing the pressure will shift the equilibrium to the left.
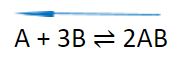
But what happens if one of the components of the reaction is in solid or liquid phase?
C (s) + CO2 (g) ⇌ 2CO (g)
In such a case, you should just count the number of molecules that are in the gas phase and ignore the ones that are in a solid or liquid phase. Accordingly, we would have 1 molecule on the left and 2 molecules on the right. Therefore, increasing the pressure would shift the equilibrium to the left since there are fewer molecules on this side of the equation. Similarly, decreasing the pressure would shift the equilibrium to the right since there are more molecules on this side of the equation.
What if the number of molecules on both sides of a reaction is the same?
A2 + B2 ⇌ 2AB
As you can see, there are 2 molecules on the left and 2 molecules on the right. For that reason, the increase or decrease in pressure will not affect the equilibrium for such reaction.
Changes in the Temperature
Last but not least, changes in temperature can also influence the position of equilibrium in a reversible reaction.
In this case, the position of equilibrium resulting from the change in temperature is dependent upon the nature of a reaction. Specifically, whether a chemical reaction is exothermic or endothermic.
In the general reactions provided below, “+Q” indicates an exothermic process where the energy/heat (Q) is released in a reaction. On the other hand, “-Q” shows an endothermic process where the energy/heat (Q) is absorbed in a reaction.
In an exothermic reaction – energy is released in forms of heat
- Increasing the temperature will shift the equilibrium to the left.
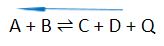
- Decreasing the temperature will shift the equilibrium to the right.
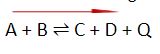
In an endothermic reaction – energy is absorbed in forms of heat
- Increasing the temperature will shift the equilibrium to the right
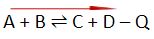
- Decreasing the temperature will shift the equilibrium to the left
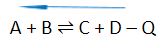
The general reactions for exothermic and endothermic processes could also be written in the following manner:
- Exothermic reaction: A + B ⇌ C + D + Q (heat is the product of a reaction)
- Endothermic reaction: A + B + Q ⇌ C + D (heat is required for the reaction to occur)
In case if there is no indication whether a reaction is exothermic or endothermic and you are simply asked to determine to which side the equilibrium would shift:
- Increasing the temperature will shift the equilibrium to the left. The reaction will attempt to absorb the heat and reach equilibrium again.
A + B ⇌ C + D
- Decreasing the temperature will shift the equilibrium to the right. The reaction will attempt to produce heat and reach equilibrium again.
A + B ⇌ C + D
IMPORTANT NOTE:
A catalyst does not impact the chemical equilibrium since it changes the rate of the forward and reverse reactions to the same extent. It will only decrease the activation energy to increase the speed of both forward and reverse reactions and reach the equilibrium state faster.
Summary
Terms and concepts defined throughout the article are summarized in the table provided below:
| Le Chatelier’s Principle | The principle enables us to qualitatively predict how the system responds to any change in its equilibrium conditions. |
| Reversible Reaction | Reactions that proceed into both forward and reverse directions simultaneously. |
| Irreversible Reaction | Reactions that only proceed into forward direction. |
| Chemical Equilibrium | The state when the concentrations of reactants and products no longer change. |
| Dynamic Equilibrium | There are no observable changes in a reaction, but the system is in constant, dynamic motion in both directions. Reactants are constantly transformed into products, and products are transformed continuously into reactants as well. |
| Factors affecting the equilibrium | ConcentrationPressureTemperature |
| A factor that DOES NOT affect the equilibrium | Catalyst |
References:
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





