Table of Contents
Key Information & Summary
- Intermolecular bonds are weak electrostatic interactions between neutral molecules and ions.
- Polarizability is the ability to form instantaneous dipoles.
- The London forces (also known as dispersion forces or instantaneous dipole-induced dipole forces) identify all those forces due to instant multipoles.
- The Debye Forces are intermolecular forces resulting from the interaction of a permanent dipole and an induced dipole.
- The intensity of Van der Waals forces varies as the intermolecular distance varies, according to the Lennard-Jones model.
- Hydrogen bond or hydrogen bridge is a special case of intermolecular force in which a hydrogen atom in a covalent bond is involved with very electronegative elements.
- Halogen bonding is a non-covalent interaction that occurs between a halogen atom, which acts from Lewis acid, and a Lewis base.
- The ion-solvent interactions are the intermolecular interactions that intervene between a solute ionic and a solvent (polar or non-polar).
Introduction to Intermolecular Forces
Intermolecular forces are weak electrostatic interactions between neutral molecules and ions. The energies involved in these types of interactions are far less intense than those involved in intramolecular chemical bonds (up to 4000 kJ/mol for an ionic bond, for example). They are always very important for evaluating the interactions between two molecules and become fundamental in fields such as reaction dynamics or drug docking.
Unlike the interatomic bonds, which bind the atoms of the same molecule together, the intermolecular forces act between two or more molecules.
Most of these forces depend on the possibility to form dipoles in a molecule. This property is called polarizability and it is the relative tendency of the electron cloud of a molecule to have its charges displaced by any external electric field. The electric field in the cases of intermolecular forces is generated by a polar molecule.
The intermolecular forces contribute to some physical characteristics of the substances, such as melting or boiling point. They are responsible of the physical state of the material. As instance, a higher force among the molecules of a solution (for example a hydrogen bond, as in the case of water) raises the boiling point, becauseto bring the molecules into the vapor phase is more expensive in terms energy.
Types of Intermolecular Forces
There are several types of intermolecular forces, arranged in order of increasing strength:
London dispersion force
London dispersion force, with binding energy between 0.05-40 kJ / mol
The forces of London (also known as dispersion forces or instantaneous dipole-induced dipole forces) identify all those forces due to instant multipoles. These forces are part of the wider category of van der Waals forces. Their name derives from the German-American physicist Fritz London.
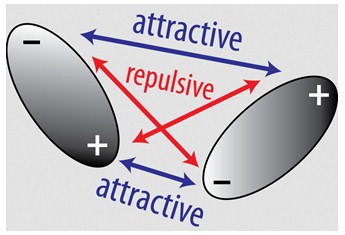
The presence of the London forces is due to the correlated movements of the electrons, a quantum effect due to the interactions between the electrons of the two interacting systems. The interaction between the electronic clouds causes their redistribution, with a consequent creation of a dipole, due to the lost symmetry between distribution of positive charges and electron cloud. The dipole allows the attraction between the two interacting systems. These forces are always present and often represent a significant part of the total interaction force, even if individually a London interaction is much weaker than an ionic bond and a hydrogen bond.
In the case of neutral atoms, such as noble gases, London's forces are the only ones present. Thanks to them noble gases also exist in the liquid form.
The forces of London increase their strength with the size of the atom or molecule, due to the greater tendency to polarize larger electronic clouds. An example case is that of halogens, which from the smallest to the largest are fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). Fluorine and chlorine are gas at room temperature, while bromine is a liquid and iodine is a solid.
Debye force
Debye force, with binding energy between 2-10 kJ / mol
The Debye Forces are intermolecular forces resulting from the interaction of a permanent dipole and an induced dipole. Debye's forces are part of the Van der Waals forces and express the induction effect.
Van der Waals forces
Van der Waals forces, with binding energy between 5-25 kJ / mol
The forces of van der Waals are named in honor of the scholar Johannes Diderik van der Waals. these forces are attractive or repulsive forces between molecules. The term "van der Waals force" includes three different types of intermolecular interactions:
permanent dipole-dipole strength (or Keesom's strength);
permanent dipole-induced dipole force (or Debye force);
induced instantaneous dipole-dipole force (or London dispersion force).
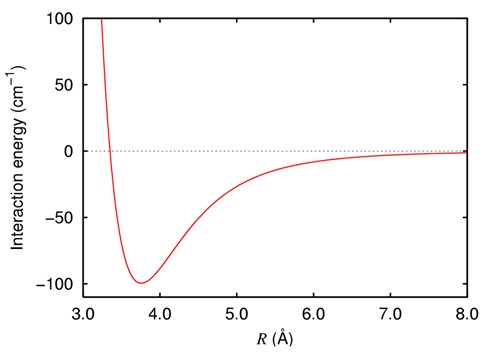
Van der Waals forces play a fundamental role in several scientific fields such as supramolecular chemistry, structural biology, nanotechnology, surface science or condensed matter physics. The van der Waals forces differ from covalent and ionic binding as they depend on the fluctuations in the distribution of charges in the molecules; these are long-range and short-range repulsive forces. All the van der Waals forces present anisotropy, which means that they depend on the relative orientation of the molecules: the induction interactions (Debye force) and dispersion (London force) are always attractive, regardless of the orientation, however the electrostatic interactions (force of Keesom) change sign with the rotation of the molecules. The intensity of Van der Waals forces varies as the intermolecular distance varies, according to the Lennard-Jones model:
Hydrogen bond
Hydrogen bond, with binding energy between 10-40 kJ / mol
Hydrogen bond or hydrogen bridge is a special case of intermolecular force in which a hydrogen atom in a covalent bond is involved with very electronegative elements (such as fluorine (F), oxygen (O), nitrogen (N)). The very electronegative element attracts the valence electrons, acquiring a partial negative charge (δ-) leaving the hydrogen with a partial positive charge (δ +). At the same time, hydrogen is attracted to an electronegative atom of a nearby molecule.
An important observation is that it is highly directional: the molecules must be aligned along the same axis to have a maximum strong bond, otherwise they will result in a lower force bond. This can be of crucial importance in the DNA, in which there are many hydrogen bonds between the nucleic acids, or in the folding of proteins where the different intensity of these interactions gives shape to a precise conformation of the protein, that is able to make it functional.
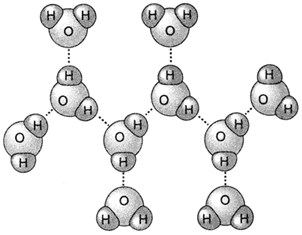
The hydrogen bond is present in both liquid and solid state water, and is responsible for its relatively high boiling temperature (when compared, for example, to H2S, which, although having a higher molecular weight, is significantly less polar). In particular, without the contribution of hydrogen bonds, the water would boil at -140 ° C.
In the solid state, a water molecule is bound through hydrogen bonding to four other molecules. The hydrogen bond happen and is stabilized to a certain intermolecular distance, longer than the other forces. In the liquid state this structure is demolished and the molecules are no longer forced into an expanded structure like in the solid, this is why the ice is less dense than water. The temperature in fact induces the breaking of some hydrogen bonds present in the ice and this allows the molecules to come closer.
Read more about the shapes of molecules and ions
Halogen bond
Halogen bond, with binding energy between 5-180 kJ / mol
Halogen bonding is a non-covalent interaction that occurs between a halogen atom, which acts as Lewis acid, and a Lewis base.
It is possible to understand the halogen bond by comparing it with the most common hydrogen bond:
Hydrogen bond: D ··· H-B
Halogen bond: D ··· X-B
In both cases, both the hydrogen and the halogen atoms act as electron acceptors for an atom or group D representing the donor. By establishing their covalent bond with B, both hydrogen and halogen tend to assume a positive partial charge: more electronegative will be the species linked to halogen (or B), the greater the possibility of obtaining stronger halogen bonds.
Ion-dipole interaction
Ion-dipole interaction, with binding energy between 40-600 kJ / mol
The ion-solvent interactions are the intermolecular interactions that intervene between a solute ionic and a solvent (polar or non-polar).
An example of such interactions are those occurring between the sodium chloride ions (NaCl) and the water molecules (H2O) when a grain of salt is dissolved in a glass of water.
During the solvation phenomenon, in addition to ion-solvent interactions, ion-ion interactions also occur.
Frequently Asked Questions
How do you define intermolecular forces?
Intermolecular forces are the weak forces of attraction present between the molecules which hold the molecules together.
What are the different types of intermolecular forces?
The main types of intermolecular forces are the London dispersion force, Debye force, Van der wall forces and hydrogen bond.
Which state of matter has the strongest intermolecular forces?
Solids have the strongest intermolecular forces due to which they exist in hard and rigid forms.
Which intermolecular force is weakest?
London dispersion force is considered to be the weakest among different intermolecular forces. However, it can become significant in larger molecules.
References and further readings:
https://www.youtube.com/watch?v=FfvG46ZmtWQ
Kenneth W. Whitten-General Chemistry - Textbook Only-Cengage Learning (2000)
https://sciencing.com/covalent-vs-hydrogen-bonds-5982030.html





