Table of Contents
Summary
- Infrared spectroscopy is used to identify various functional groups present in the chemical compound.
- The mid infrared spectroscopy is concerned to the vibrational energy (and rotational energy) of the molecule.
- The region of great interest and practical use for the organic chemist is the mid infrared (4000 – 400 cm-1) that provides important information about the molecular structure and functional groups present in the molecule.
- IR spectroscopy can be used both for qualitative and quantitative analysis.
Infrared Spectroscopy
Spectroscopy is the science of the interaction of electromagnetic radiation with matter. This interaction provides valuable information about the structure of molecules. Electromagnetic radiations are classified by their wavelength or frequency into radio waves, microwave, infrared, visible, ultraviolet, X-rays, and gamma rays. Here we study infrared radiations and the information obtained by their interactions with molecules.
The infrared radiations refer to the part of the electromagnetic spectrum between the visible and microwave region. This spectral region is generally subdivided into three portions, near-infrared (14,290 – 4000 cm-1), mid-infrared (4000 – 400 cm-1), and far-infrared (700 – 200 cm-1). The range of numbers represents wave number (cm-1). The region of great interest and practical use for the organic chemist is the mid-infrared (4000 – 400 cm-1) that provides important information about the molecular structure and functional groups present in the molecule. Also, the energy of most molecular vibrations corresponds to that of the infrared region of electromagnetic radiations.
A simple molecule on interaction with IR radiations gives a complex plot of spectra. The organic chemist may utilize this information in elucidating the structure of the molecule by having a concise inspection of peak to peak correlation. The intensity of absorption or peak height could be studied for quantitative analysis.
Interaction of Matter with IR radiation: Basic principle
Atoms in the molecule are in a constant state of motion and each of them poses three degrees of freedom. All these movements confer the atom a quantified amount of energy. The total atomic energy is the sum of electronic energy (E elec), vibrational energy (E vib) , and rotational energy (E rot).
E tot = E elec + E vib + E rot
The interaction of electromagnetic radiation with a molecule causes a change in the total energy of the system.
Two different atoms bonded to each other at the two extremes of a chemical bond, they form an electric dipole. Such a chemical bond can be called a non-symmetrical bond. If such a non-symmetrical bond is irradiated by electromagnetic radiation whose frequency is the same as the dipole, the electrical component of the electromagnetic radiation could transfer its energy to the bond resulting in energy absorption. The radiations from the mid-infra-red region possess low energy but are sufficient to cause vibrational and rotational excitation. This energy interaction causes changes in vibrational and rotational modes of electrons of the bonded atoms. The electrons absorb energy from the incident radiation and excite from lower energy level to the higher energy level. Molecules, where there is no change in dipole moment, no change in vibrational energy (movement), is observed.
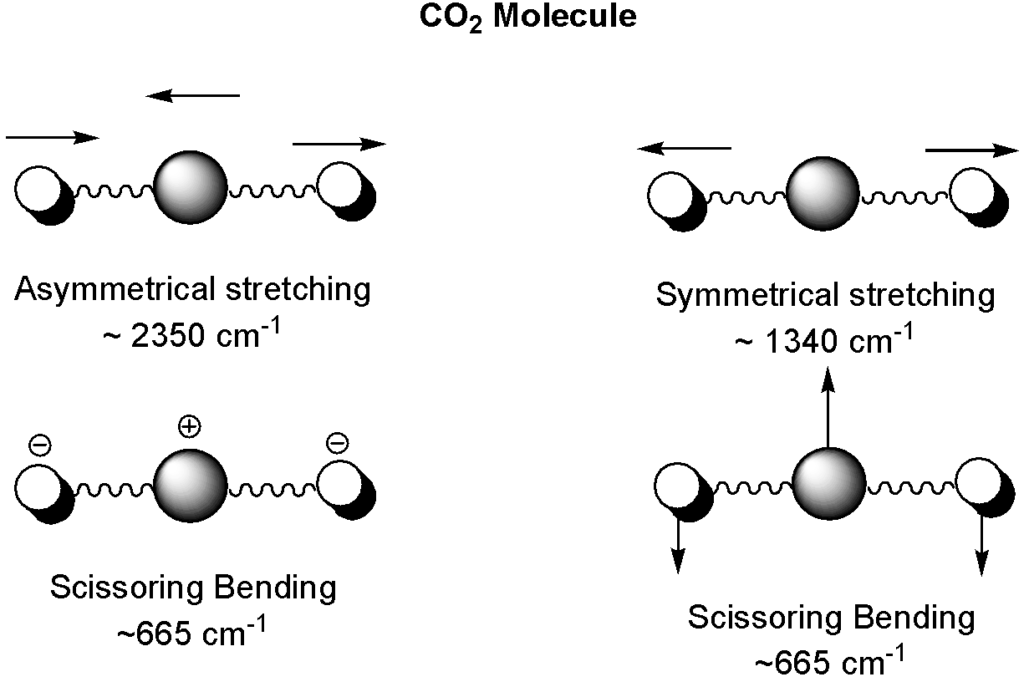
In the mid-infrared spectroscopy, we are concerned with the vibrational energy (and rotational) of the molecule. There are two types of molecular vibrations, stretching and bending. A stretching vibration is a rhythmical movement with changes in the interatomic distance along the bond axis while bending movement involves a change in bond angle. Bending might be twisting, rocking, scissoring, and wagging. In a simple molecule such as carbon dioxide, these vibrations can be presented as
The main components of a FT – IR spectrophotometer are
- Light source
- Interferometer
- Detector
Light Source
The commonly used IR radiation sources for IR spectrophotometer are inert solids, generally, zirconium oxide or rare earth oxides (Nernst glower, Globar made of silicon carbide) and nichrome coil. IR source is heated to 1000 – 1800 0C. It produces continuous radiations. In FT – IR the source is cooled with water to provide better power and stability
Interferometer
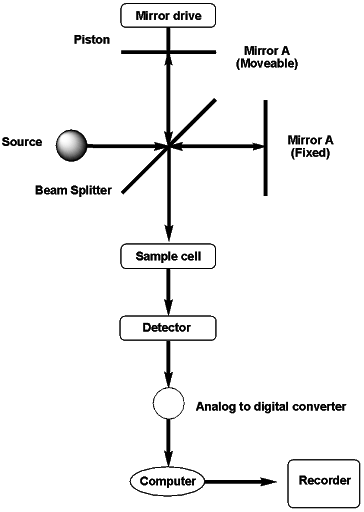
The most commonly used interferometer is Michelson. It consists of three components, a fixed mirror, a moveable mirror, and a beam splitter. The two mirrors are perpendicular to each other. The incident light from a polychromatic source impacts on a beam splitter. This beam splitter is made of a semi-transparent film of germanium deposited on potassium bromide. The beam splitter divides the incident beam into two halves. One part is directed towards the fixed mirror while the other is towards the moveable mirror. The distance of the light beam varies from the beam splitter. Due to changes in the relative position of the moveable and fixed mirror, an interference pattern is generated. The recombined beams follow the same path passing through the sample and reaching the detector.
Detector
Various detectors are used in IR spectroscopy. In FT-IR instruments, the generally used is a pyroelectric detector and photodiodes and diodes arrays. The pyroelectric detector contains monocrystals of deuterated triglycine sulfate or lithium translate sandwiched between two electrodes.
Fourier transform infrared spectroscopy (FT – IR)
Fourier transform spectroscopy is a measurement technique whereby spectra are collected based on measurements of the temporal coherence of a radiative source, using time-domain measurements of electromagnetic radiation or other types of radiation. FT-IR spectrometer contains a single beam optical assembly with an essential component, an interferometer. This interferometer is generally of Michelson type, placed between source and sample.
There are a number of advantages of FT – IR technology.
- As the incident light is not passed through the monochromater, so the entire wavelength range pass through the sample in one time and thus much time is saved.
- Data goes from analogue to digital conversion, leading to easy result processing
- Results of various scans are combined to average out giving us excellent spectra from very small sample
Attenuated Total Reflectance (ATR)
ATR is an IR sampling technique that enables to analyse the solid and liquid samples directly by avoiding complex sample preparations. ATR operates through total internal reflection by measuring changes in the infrared beam. The infrared beam is directed onto an optically dense crystal having a high refractive index at a certain angle in such a way that it reflects at least once off the internal surface that is in contact with the sample. This internal reflection forms an evanescent wave. The beam on its exits from the ATR crystal is then analysed by the detector. The succession of several attenuated reflections leads to an effective optical pathway comparable to that obtained through conventional transmission.
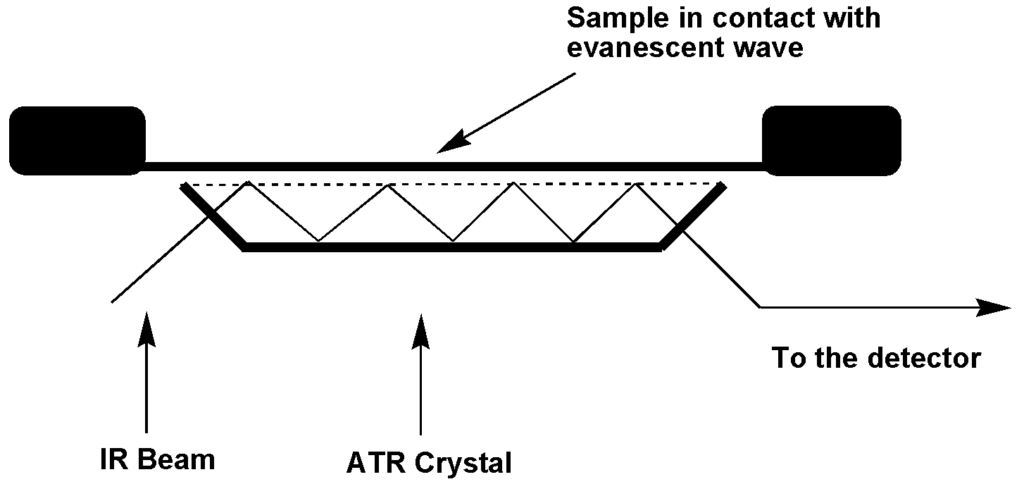
The crystal material must be transparent in the IR region. The typical materials used are germanium, Zinc selenide (ZnSe), and diamond. Diamond is commonly used because of its excellent mechanical properties. The ATR sampling techniques offer a lot of advantages in IR spectroscopy, such as:
- Faster sampling avoiding complex preparations
- Greater reproducibility of results
Minimum user interference and user to user variations
Sample preparation
Compounds can be examined in solution, in a gaseous state, like pure liquid or solid as such.
Gas samples
Gas samples can be analyzed in an IR spectrophotometer directly. The sample is enclosed in a small special cell usually about 10cm that is placed directly in the path of incident radiation. Cells with longer path lengths are also in use. The two ends of the cell is usually made of sodium chloride which is transparent in the IR region and does not absorb.
Liquid samples
A drop of a pure liquid is placed between the sodium chloride plates and examined.
Solution samples
Solid is dissolved in carbon tetrachloride or chloroform at usually 1-5% concentration level. The solution is placed in a special cell of sodium chloride.
Solid sample
Finely grounded solid is mixed with hydrocarbon liquid such as nujol or hexachlorobutadiene and a paste is made of it. This paste is placed between flat plates of sodium chloride that are placed in the path of incident IR radiations in an IR spectrophotometer. Alternatively, the material is mixed with potassium bromide and pressed to form a disc that is subsequently placed in the spectrophotometer.
Important to note, the spectrum of the same compound in solid, solution, or liquid form might be slightly different due to different molecular interactions in solid and liquid states.
ATR sampling techniques
ATR technique is a new sampling approach in IR spectroscopy. This technology advancement brought significant ease to the recording of the IR spectrum. The sample does not require any pre-mixing or preparations. The solid or liquid sample is directly placed at ATR crystal to record IR spectrum. With the ATR set up, we can record the spectrum of various other materials under study e.g. fibers can be studied for any dye uptake or chemical treatment. Hair fibers can be examined directly for protein damage.
Interpretation of IR Spectra
There is no single perfect rule for the interpretation of the spectrum. The basic requirements or precautionary measures must be entertained interpret the spectrum
- Sample handling must be specified
- Chemical compound must be of reasonable purity
- Spectrum should be of adequate resolution and intensity
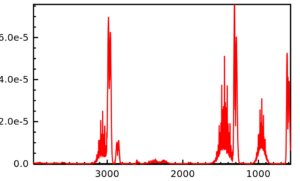
An IR spectrum is generally a complex plot. Different groups have absorptions at a wide range of wavelengths. A careful empirical interpretation is required by comparing the spectrum with already available knows standards. A peak-to-peak correlation with known values of IR absorptions for a given functional group or particular bond can guide scientists towards the identification of the chemical structure of an unknown chemical compound.
As an example carbon dioxide spectrum is
Application of Infrared spectroscopy
Infrared spectroscopy is used for the detection and quantification of various chemicals in different scientific and industrial operations.
Food analysis
IR spectroscopy can be utilized to detect and measure additives and preservatives in foods. Adulteration of juices, vegetable oils, and milk, as well as caffeine content, protein, and lipid content, or sugar composition are other examples that have been reported.
Analysis of edible oils
Analysis of edible oils and fats is an important activity in the food industry. Quantification of cis and trans unsaturation levels is carried out using infrared spectroscopy. Edible oils that are in cis form are liquids. During hydrogenation of these oils, some cis bonds may get converted to trans. As we know, trans unsaturated fatty acids are reported to be unhealthy and may lead to heart disease. trans has a unique stretching at 966 cm-1 that can be used to detect and even quantify using an infrared spectrophotometer.
Pulp and Paper Industries
Infrared spectroscopy is employed for quality control in the pulp and paper industries. Paper is comprised of cellulose fibers, inorganic fillers, and binders. The addition of polymers and calcium carbonate may be identified by an IR in paper. Any modification in cellulose can also be detected using infrared spectroscopy.
Environmental Applications
Infrared spectroscopy has been applied to a broad range of environmental analyses, including air, water, and soil analysis. Other common applications include industrial gas emissions, emissions from fires, and astronomical applications. Determination of the compositions of atmospheric gases is important for an understanding of global climate changes and infrared spectroscopy can be used for measuring the most abundant and important greenhouse gases, i.e. CO2, CH4, and N2O.
Forensic analysis
Forensic science employs infrared spectroscopy in examining the specimens under investigation. ATR technique has made great achievement for this because it requires a small amount of sample and is non-destructive. Analysis of blood or pigment stain over clothes or fabric or other materials can be analyzed easily. Also, hair fiber can be examined for any contamination.
Frequently Asked Questions
What is the principle of infrared spectroscopy?
The basic principle of infrared spectroscopy is that molecules absorb different frequencies of infrared radiations determined by their specific structure.
Why is infrared spectroscopy used?
Infrared spectroscopy is used to determine the structure of molecules. It helps to find the vibrational energy of molecules and the different functional groups in them.
What are the types of infrared spectroscopy?
Infrared spectroscopy is of two types: Fourier transform infrared spectroscopy and dispersive infrared spectroscopy.
What are the components of flourier transform infrared (FT-IR) spectrophotometer?
The main components of an FT-IR spectrophotometer are a light source, interferometer and detector.
Books for further study
- Rouessac, F. and A. Rouessac. Chichester: Chemical analysis, modern instrumentation, methods and techniques. John Wiley & Sons Ltd. 2007.
- Silverstein, R. M., F.X. Webster, and D.J. Kiemle. Spectrometric identification of organic compounds. Hoboken. John wiley & sons, Inc. 2005.
- Frank A. S: Handbook of instrumental techniques in analytical chemistry. (City) Upper saddle river, 1997, Prentice Hall PTR.
- Sturat, B. Infrared spectroscopy, fundamentals and applications. John wiley & sons.
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





