Table of Contents
Key Information & Summary of Halogenoalkanes
- The halogenoalkanes (or alkyl halides) are a group of organic compounds derived from alkanes containing one or more halogens.
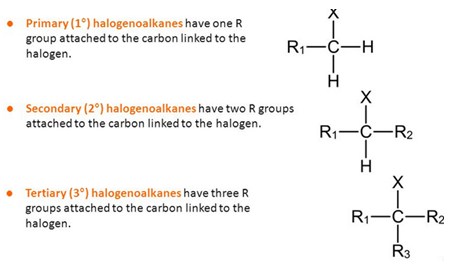
- Halogenoalkanes can be classified according to their structure. A name is given considering the connectivity of the carbon atom to which the halogen is attached.
- They are colourless, relatively odourless, and hydrophobic.
- The melting and boiling points of chloro-, bromo-, and iodoalkanes are higher than the analogous alkanes, scaling with the atomic weight and number of halides.
- Some halogenoalkanes are used ar fire extinguishers because they contain fewer CH bonds so are less flammable than alkanes.
- The halogenoalkanes can be obtained from alkane, alkenes, alkynes and alcohol
- The alkyl halides are very useful in organic synthesis in the reaction with metals to organometallic compounds, especially lithium and magnesium (Grignard reagents).
- Hydrolysis of halogenoalkanes can give the alcohols in presence of a base.
The halogenoalkanes (or alkyl halides) are a group of organic compounds derived from alkanes containing one or more halogens. They have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).
These compounds are generally used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals.
The chlorofluorocarbons have been banned from the environmental protection agencies because they have shown to lead to ozone depletion. Halogenoalkanes which contain chlorine, bromine, and iodine are generally thread to the ozone layer, but fluorinated volatile haloalkanes are supposed to be greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties.
Halogenoalkanes are also found in nature. Bacteria produce them through enzyme-mediated synthesis but also fungi, and especially sea macroalgae can create halogenoalkanes.

Classes
Halogenoalkanes can be classified according to their structure. A name is given considering the connectivity of the carbon atom to which the halogen is attached. In primary haloalkanes, the carbon connected to the halogen atom is only attached to one other alkyl group. An example is chloroethane (CH3CH2Cl).
In secondary haloalkanes, the carbon connected to the halogen atom has two C–C bonds.
In tertiary haloalkanes, the carbon that carries the halogen atom has three C–C bonds.
Haloalkanes containing carbon bonded to fluorine, chlorine, bromine, and iodine are called organofluorine, organochlorine, organobromine and organoiodine compounds, respectively.
Compounds containing more than one kind of halogen are chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs).
Properties
Halogenoalkanes has properties that are similar to the parent alkanes. They are colourless, relatively odourless, and hydrophobic. The melting and boiling points of chloro-, bromo-, and iodoalkanes are higher than the analogous alkanes, scaling with the atomic weight and number of halides. The increasing strength of the intermolecular forces (London dispersion to dipole-dipole interaction) is responsible for this effect. Thus carbon tetraiodide (CI4) is a solid whereas carbon tetrachloride (CCl4) is a liquid. This is just a general consideration and as usual there are exceptions. Certain fluoroalkanes have lower melting and boiling points than their non-halogenated analogues. This is due to the fact that a fluorine has lower polarizability. For example, methane (CH4) has a melting point of -182.5°C whereas tetrafluoromethane has a melting point of -183.6 °C .
Some halogenoalkanes are used as fire extinguishers because they contain fewer CH bonds so are less flammable than alkanes. Halogenoalkanes are characterized by an increased polarizability compared to the parent alkanes, thus halogenoalkanes are better solvents than the corresponding alkanes.
Also reactivity is increased by the presence of a halogen (except fluorine) respect to the parent alkanes.
Synthesis
The Halogenoalkanes can be obtained from:
- alkenes and alkynes by addition of a hydrogen HX, the electrophilic attack of hydrogen is followed by the attack of the halogen;
- alcohols by reaction with SOCl2, thionyl chloride acts as a dehydrating agent by promoting an intramolecular nucleophilic attack of chlorine, leading to the formation of alkyl halide and to the release of gaseous SO2;
- alkanes, generally an alkane and a halogen are reacted to heat and a halide plus a hydrogen H-X is obtained.
- Finally, halogenoalkanes can be obtained by electrophilic substitution from aromatic hydrocarbons.
Reactivity
The alkyl halides are very useful in organic synthesis. One of their most important uses in the chemical laboratory is in the reaction with metals to organometallic compounds, especially lithium and magnesium (Grignard reagents).
The reaction is normally carried out by magnesium or metallic lithium with an alkyl halide solution.
CH3CH2Cl + Mg (ether) -------> CH3CH2MgCl
This is a heterogeneous reaction, then occurs at the interface between the solid phase (magnesium, lithium) and liquid (halide solution in ether).
Preparing Grignard reagents, magnesium is used in powder. For the reaction it is possible to use bromides, iodides and chlorides; however, bromides are generally used due to the high cost of iodides and for the low reactivity of chlorides.
The reaction is strongly exothermic and must be carried out under anhydrous conditions, to avoid the reaction with water. This reaction would consume the reagent by converting it into the corresponding alkane:
CH3CH2MgBr + H2O ------> CH3CH3 + HOMgBr
Organolithium reagents are prepared in the same way using lithium instead of magnesium.
Hydrolysis of halogenoalkanes can give the alcohols if the hydrolyzing agent is not a strong enough base to give the competing elimination reactions. To minimize this side reaction, a weak base, in the form of dilute aqueous sodium hydroxide, is used.
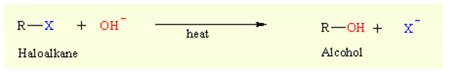
The ease with which this reaction occurs depends on the C-X bond strength:
R-I > R-Br > R-Cl >> R-F
Frequently Asked Questions
What are the halogen alkanes?
Halogen alkanes are the derivatives of alkanes in which one or more hydrogen atoms of alkanes are replaced by halogen, such as CH3Br, CH2Cl2, CH3CH2Cl etc.
How are halogen alkanes classified?
Halogen alkanes are classified as primary haloalkane if the halogen-bound carbon atom has one C-C bond; secondary haloalkanes if the halogen-bound carbon atom has two C-C bonds, and tertiary haloalkanes if the halogen-bound carbon atom has three C-C bonds.
How is an alcohol produced from halogen alkanes?
Alcohol is produced from halogen alkanes by reacting them with water.
R-X + H2O → R-OH + HX
What are the physical properties of halogen alkanes?
Halogen alkanes are usually colourless and odourless compounds. They are hydrophobic and only slightly soluble in water. The boiling point of halogen alkanes is usually greater than the parent alkane if the number of carbon atoms remains the same.
References and further readings:
https://chemstuff.co.uk/unit-2/functional-groups/halogenoalkanes/
https://www.slideshare.net/aalekhpatel/halogenoalkanes-as-level-chemistry
https://www.chemguide.co.uk/organicprops/haloalkanemenu.html
https://en.wikipedia.org/wiki/Haloalkane
https://study.com/academy/lesson/nucleophilic-substitution-in-haloalkanes.html





