Table of Contents
Summary
- Crude oil is a complex mixture of various organic compounds.
- Fractional distillation is the process to separate this mixture into its component parts or fractions.
- The organic molecules due to their different molecular structures & weights condense at different temperatures.
- The process is carried out in a fractionating tower which is steadily cooler towards the top.
- The large molecules with higher molecular weight stay in the bottom while smaller molecules with low molecular weight condense at top.
- Major fractions obtained are paraffins, naphthalenes, aromatic and organometallic compounds
- This process converts crude oil of less economic importance into more valuable and usable products.
- Well-known fractions are gasoline, diesel oil and kerosene that are used as engine fuel for our cars, trucks and even for jet engines.
- Keep reading for more facts about the fractional distillation of crude oil.

Petroleum is a major source of energy and since its discovery, its demand is ever rising. We need the energy to move our vehicles, keep our homes warm and do all this; we use natural gas, gasoline, or diesel. These all are extracted from crude oil that is available in large reservoirs underneath the earth's crust. The black thick liquid with a special odour extracted from underground reservoirs is called crude oil or petroleum. Humans discovered oil usage thousands of years ago and started its extraction for fuel consumption. In the early days, the extraction and processing techniques were relatively simple, however, with the passage of time and the development of new advanced technologies, crude oil processing has changed and its demand and consumption have been on the rise. Today almost every area of our life is directly linked to the petroleum industry and its prices.
Crude oil when extracted is a thick black liquid. It is a complex mixture of different chemical compounds known as hydrocarbons ranging from methane to asphalt. The composition of crude oil varies with the type of crude oil and how it is extracted. Hydrocarbons such as alkanes (saturated chemical molecules) are the main fraction of crude oil. It may also contain aromatic compounds and other compounds with nitrogen, sulfur along with metals such as copper, nickel, vanadium, and iron. Hence crude oil is the main source of these organic compounds and fractional distillation is carried out to make these organic compounds available and usable.
Basic principle of fractional distillation
Organic molecules vary in their physical and chemical properties due to their different carbon chain lengths and molecular weights. The melting point, boiling point, viscosity and colour all are related to the molecular weight and structure of the molecule. The boiling point of organic compounds increases with increasing molecular weight. The molecule with a large number of carbons has a higher molecular weight and higher boiling points. As mentioned earlier, crude oil contains a wide range of organic compounds that differ in their carbon chains and boiling points. Fractional distillation is the process to separate these individual fractions from each other. The whole process is based on the principle that different substances boil at different temperatures. For example, crude oil contains kerosene and naphtha, both are useful fractions but have different usage and applications. Naphtha can be used to form gasoline (Petrol) for cars while kerosene is used for jet fuel. Both have different carbon chain lengths and structures. During distillation, the mixture of kerosene and naphtha is first vaporized then they are cooled down; the kerosene condenses at a higher temperature than the naphtha. As the mixture cools, the kerosene condenses first, and the naphtha condenses later. This is how fractional distillation works. The whole process can be divided into steps.
Read more about Organic Synthesis
Pretreatment step
The crude oil extracted from the oil well could be an emulsion containing some rock salt and water. In the first step, crude oil is pre-treated to remove this water and salt (Sodium chloride or brine). The desalting step is important because the presence of sodium chloride can generate highly corrosive hydrogen chloride gas during the distillation process. This hydrogen chloride being a strong acid may cause severe damage to the processing equipment. The desalting can be carried out by heating the crude oil under pressure or adding demulsifying agents e.g. soap, fatty acids, and surfactants to break the emulsion.
Distillation
The crude oil is heated to 350oC or above using heat exchangers and is converted to vapours. These vapours are charged to the distillation tower and they travel from bottom to top. The whole process is typically conducted in a vertical tower or column ranging from 0.5 to 6.0 meters in diameter and 6-60 meters in height. The tower has a temperature gradient, which means it is hot at the bottom and cooler at the top. It contains a series of trays installed to collect fractions that are finally removed via outlets. Because different fractions have different boiling points, they condense at different heights at different levels of the column. The large molecules with higher molecular weight stay in the bottom while smaller molecules with low molecular weight travel upward and condense at their respective collection tray. The solid waxy residue is removed from the bottom while molecules with very low molecular weight stay as gases and are collected from the top. They are called petroleum gas.
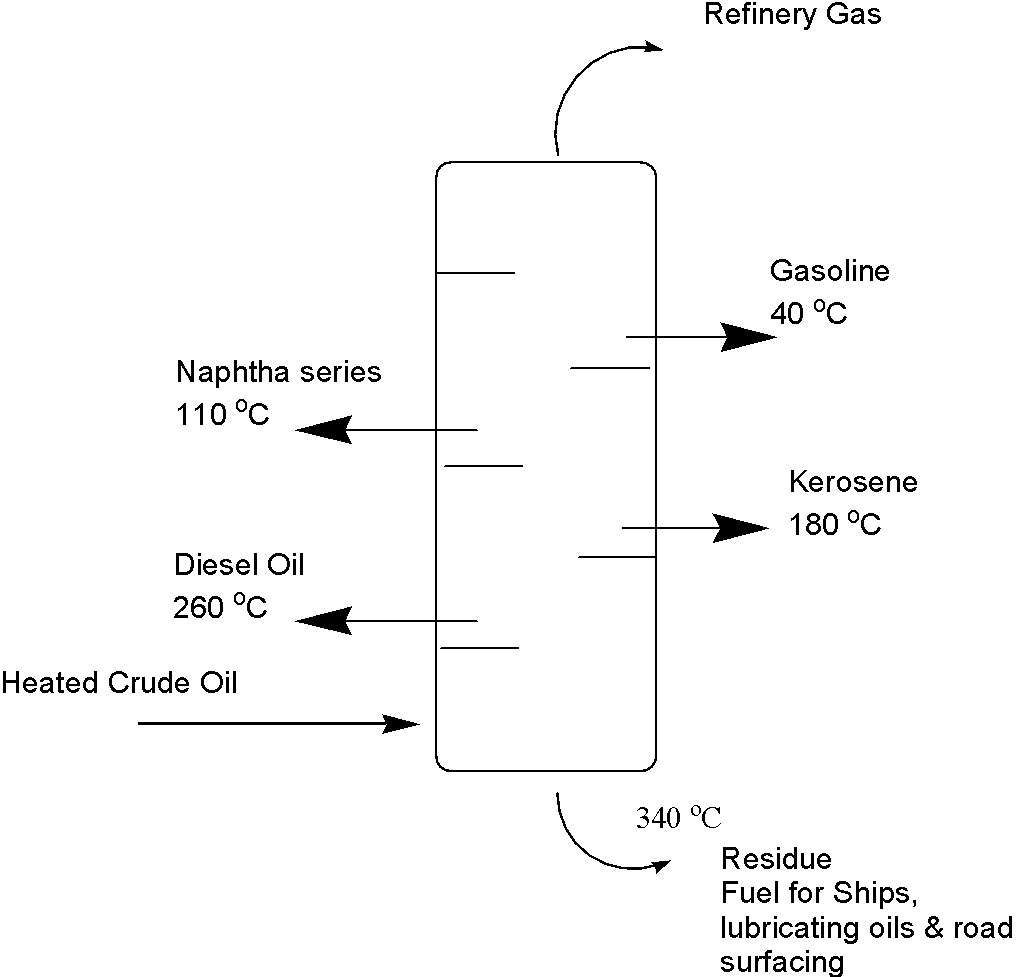
Different Fractions obtained by distillation
The two major groups of a class of organic compounds obtained from distillation are hydrocarbons and hetero-atomic compounds. Among hydrocarbons, the fraction distillation gives paraffins, Naptha, unsaturated aliphatic, and aromatic compounds.
Paraffins
Paraffins are saturated hydrocarbons where a carbon atom is attached to four other separate atoms. The general formula for such a class of compounds can be described as (CnH2n+2) where n is the number of carbon atoms in the compound. They are also called alkanes. They are the major fraction obtained from crude oil (30-60%). They may range from simple molecules such as methane, ethane, propane, and butane to longer carbon chain molecules such as Hentriacontane (C31H64). These molecules are stable and due to saturation are less reactive among other hydrocarbons. Paraffins could be straight-chain (n-paraffins) or branched-chain molecules (iso-paraffins).


Figure 1: (A) n-paraffin, (B) Isooctane (2,2,4-Trimethylpentane).
n-paraffins have poor anti-knocking properties compared to their branched-chain analogues (iso-paraffins). Branched-chain fractions perform better in the internal combustion engines and hence are more in demand. This drives a series of chemical modifications to convert n-paraffins to iso-paraffins by catalytic reforming, alkylation, polymerization, or isomerization.
Gasoline or petrol also belongs to this class and is the major light vehicle fuel. It is branched-chain octane mixed with anti-knocking agents and octane enhancers. Kerosene and famous diesel oil are all predominantly paraffins.
Alkenes
Alkenes or olefins are actually not present in crude oil. They are made during petroleum processing via cracking. Their general formula is CnH2n. They are reactive molecules as they have a double bond in their chemical structure. They easily undergo oxidation and hence improve the anti-knocking quality of finished fuel. Due to their reactivity, they are utilized to synthesize various other organic compounds used in various other chemical industries. Such chemicals are generally called petrochemicals.
Napthalene series
They are saturated cyclic organic compounds also known as cyclic alkanes. Their general formula is the same as alkenes, CnH2n. They are the second most abundant fraction (30-50%) of crude oil. The dehydrogenation of these compounds gives a series of benzene compounds which are important precursors for other organic compounds. They are also good to stock for lube oil.

Aromatic Compounds or Benzenes
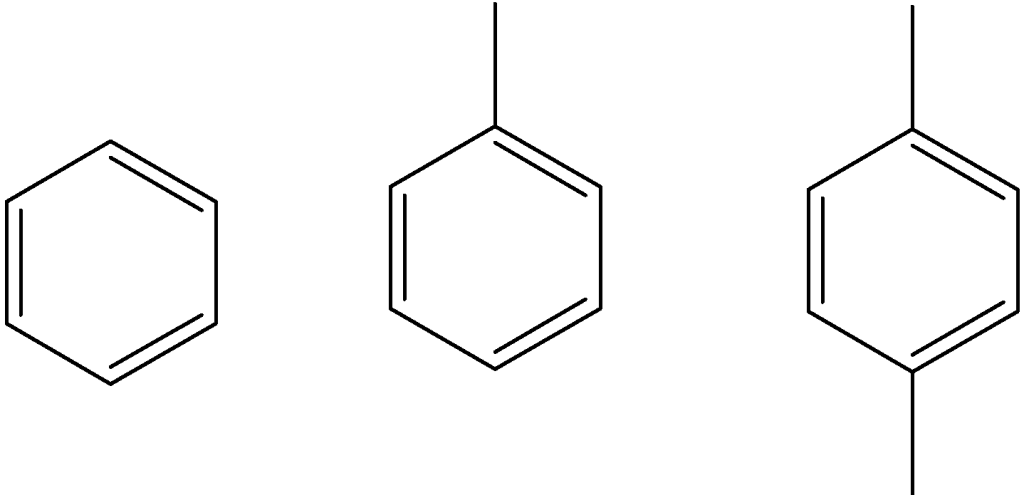
They make only a small portion of crude oil. They have a high anti-knocking value and hence are highly desirable for gasoline mixture. Their general formula is CnH2n-6. Simple aromatic compounds such as benzene, toluene, and xylene are a good sources of carbon for further organic synthesis f petrochemicals.
Hetero-Atomic Compounds
The most common hetero-atomic class in crude oil is sulfur-containing compounds. They are present in crude oil as mercaptans, mono- and disulfides with the general formula R-SH, R-S-R1, R-S-S-R1, where R and R1 are the alkyl radicals. Cyclic sulfur compounds e.g. thiophenes and benzothiophene are also obtained from crude oil. Sulfur present in petroleum fuel products may form various oxides of sulfur (SOx) during combustion, which are strong environmental pollutants.
Nitrogen-containing compounds are also present in small amounts in crude oil. They are responsible for the colour. Nitrogen in petroleum fuels causes the generation of oxides of nitrogen (NOx), which are also strong pollutants in the atmosphere. Nitrogen can be removed from petroleum products by catalytic hydrogenation.
Crude oil may also contain metallic compounds of vanadium, nickel, lead, arsenic, etc. Vanadium and nickel are found in the form of organometallic compounds mostly in the heavier fractions of crude oil. Petroleum fuels containing these metallic compounds may damage the burners, lines, and walls of the combustion chambers and hence must be removed.
Small amounts of oxygen-containing organic compounds are also obtained from crude oil. They are phenols, cresols, organic acids, etc.
Frequently Asked Questions
What is fractional distillation?
Fractional distillation is a process of separating crude oil into its component fractions.
What is the basic principle of fractional distillation?
Organic compounds in crude oil have different chemical structures and chain lengths. They boil at different temperatures depending upon their molecular structure and molecular weight. The low boiling fraction vaporizes 1st while high boiling fractions vaporize later on. On cooling, they liquify at different temperatures making their separation possible.
Which fraction is obtained at the top of the column?
The compound with the lowest boiling point is obtained at the top of the column in fractional distillation.
What are the major fractions obtained in fractional distillation?
Crude oil is separated into the following fractions in fractional distillation; gasoline, naphtha series, kerosine oil, diesel oil, and residual oil.
References
Austin, G. T. (1984). Shreve's chemical process industries. In Shreve's chemical process industries. McGraw-Hill (p 713-746).
Speight, J. G. (2014). The chemistry and technology of petroleum. CRC press.
Morrison, R. T., and R. N. Boyd. "Organic chemistry 5th edition." (1987): (p 92-95).
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





