Table of Contents
To understand what equilibrium constants are, let's start at the beginning. Chemical equilibrium is the state that indicates that the concentrations of reactants and products no longer change in a reaction. When a chemical equilibrium is a result of two active and opposing reactions, the equilibrium that is reached by the system is called a dynamic equilibrium.

Dynamic equilibrium refers to the state when the changes are no longer observable, but the system is in constant dynamic motion, meaning that the reactants are rapidly transformed into products and vice versa. The system is considered a closed system meaning that no components leave or enter the system.
Chemical reactions that proceed simultaneously in two directions (forward and reverse) are called reversible reactions.
The general equation for the reversible reaction is the following:

In a reversible reaction at equilibrium, the reaction rate for the forward and reverse reactions is the same. Moreover, concentrations of the reactants and products no longer change and stay constant.
NOTE that even though the concentrations are constant in a reaction at an equilibrium state, it does not mean that the concentrations are equal. The concentration of reactants might be more than the concentration of the products or vice versa.
Let’s consider the following figure that represents the equilibrium state of a general reaction “A+B????C.”
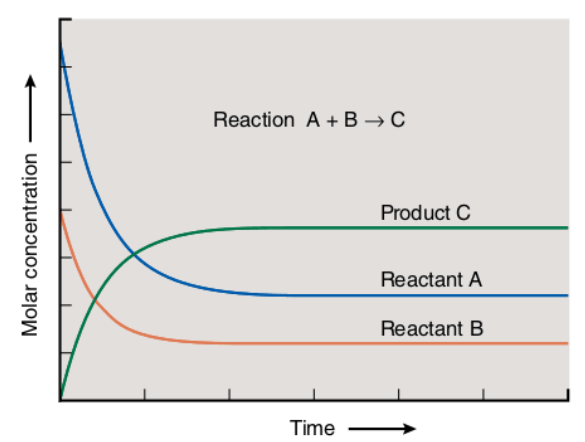
We can see that the concentration of reactants is decreasing as time elapses. On the other hand, the concentration of products increases. At some point, the concentrations of reactants and products remain constant, meaning that the reaction has reached its equilibrium state.
Learn more about Dynamic Equilibrium
Equilibrium Constants
In order to quantitatively analyze the equilibrium state of a reaction, we can use the equilibrium constant, Keq, or Kc, which is a function of the molar concentrations of reactants and products. The equilibrium constant determines the number of products relative to reactants at equilibrium. It has been shown that all chemical reactions that are at equilibrium can be described utilizing the equilibrium constant.
Consider the following general chemical equation:

Lowercase letters (a, b, c, d) indicate the coefficients used to balance the chemical equation while the capital letters (A, B, C, D) represent the substances involved in a reaction.
The equilibrium constant for the reaction above can be written as:
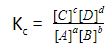
The expression of Kc is true if none of the components is a pure solid or liquid. Only reactants and products in the gas phase or solutions appear in the expression.
It is important to distinguish two types of equilibria in order to derive a proper expression for the equilibrium constant.
- Homogeneous equilibria – reactants and products are in the same phase. All components of a reaction are in the gas phase or in solution.
- Heterogeneous equilibria – reactants and products are in a different phase. Some of the components of a reaction are in a pure solid or liquid phase.
Equilibrium Constant Rules
To write the equilibrium constant for a given reaction, you should consider several rules:
- To indicate the molar concentrations at equilibrium, we use square brackets “[ ]”;
- Concentrations of the products always appear in the numerator;
- Concentrations of the reactants always appear in the denominator;
- The molar concentration of reactants and products is raised to a power equal to the particular compound’s coefficient;
- In the case of heterogeneous equilibria – pure solids or pure liquids are not considered in the expression for the equilibrium constant (their values are simply considered to be equal to 1).
Note that you should only use a balanced chemical equation to determine the proper value for the equilibrium constant. If the equation is not balanced correctly, the value for Kc will not be correct as well since you must raise the concentration of each component to the power equal to the specific component’s coefficient in the balanced equation.
Another feature that you should always take into account is that you only use the concentrations of the components of a chemical reaction if they are in the gaseous or aqueous phase. These phases are abbreviated as follows:
A (g) – meaning that the compound A is in the gas phase
B (aq) – meaning that the compound B is in the aqueous phase (solution)
In case if you have pure solids or pure liquids, you do not account for their concentrations. Such phases are abbreviated as follows:
C (s) – meaning that the compound C is in a pure solid phase
D (l) – meaning that the compound D is in a pure liquid phase
Considering the importance of the physical states of components of a chemical reaction, it is important to indicate the phase of every reactant and product in a balanced reaction. So, if we consider that A is a gas, B is an aqueous solution, C is a pure solid, and D is a pure liquid, the chemical reaction would have the following form:

Note that such a reaction might not exist in reality. The equation was implemented to simplify the concept and better represent the importance of the indication of different phases in a chemical reaction.
There are several variables that impact the value of the equilibrium constant. One of the variables is considered to be temperature. The impact of temperature change depends on whether a chemical reaction is exothermic or endothermic.
In the case of an exothermic reaction, the increase in temperature will decrease the value of the equilibrium constant.
In the case of an endothermic reaction, the increase in temperature will also increase the numerical value of the equilibrium constant.
Generally, the temperature of a reaction is considered to be constant when you are asked to calculate the equilibrium constant of a given reaction.
As we have considered a general chemical equation to define the concept of an equilibrium constant, we can move to some actual examples.



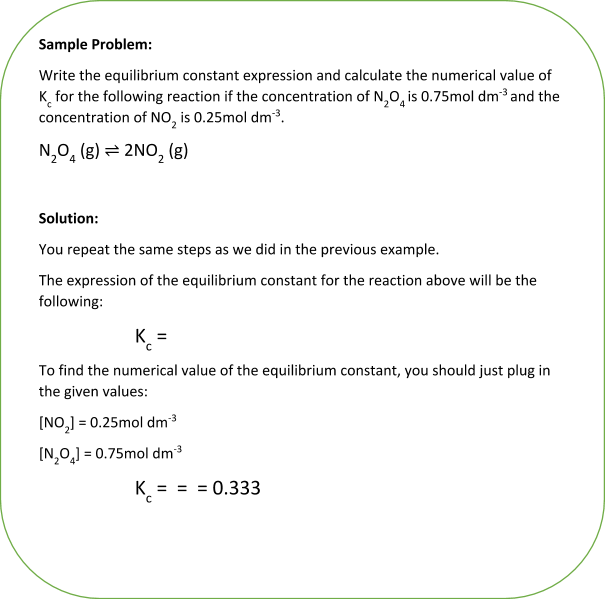
In the previous two examples, you were asked to write the expression for the equilibrium constant. You might also be asked to calculate the numerical value of the equilibrium constant for a given reaction.
Summary of Equilibrium Constants
Terms and concepts defined throughout the article are summarized in the table provided below:
| Chemical Equilibrium | The state when the concentrations of reactants and products no longer change. |
| Dynamic Equilibrium | There are no observable changes in a reaction, but the system is in constant, dynamic motion in both directions. Reactants are constantly transformed into products, and products are constantly transformed into reactants as well. |
| Reversible Reaction | Reactions that proceed into both forward and reverse directions simultaneously. |
| Irreversible Reaction | Reactions that only proceed in a forward direction. |
| Homogeneous Equilibrium | A reaction in which the reactants and products are in the same phase. |
| Heterogeneous Equilibrium | A reaction in which reactants and products are in different phases |
| Equilibrium Constant | The value is derived using the concentrations of reactants and products in a reaction at an equilibrium state. |
| Calculation of equilibrium constant | Kc = [products][reactants] = [C]c[D]d[A]a[B]b |
Note that if you are asked to calculate the equilibrium constant for a heterogeneous reaction the products of which are pure solids or pure liquids, you simply write 1 in the numerator and divide it by the concentration of the reactants.
Another important thing that you should always keep in mind is that if H2O is in a liquid phase, the value of its concentration equals 1. But if H2O is in the gas phase, you must consider the given value of its concentration.
When students see H2O, they usually do not pay attention to the indicated phase, and this is a quite common mistake. ALWAYS look at the indicated phase to identify whether the reaction involves liquid water (l) or water vapor (g).
Frequently Asked Questions
What is the equilibrium constant?
The equilibrium constant is a mathematical ratio of the concentration of products divided by the concentration of reactants at the equilibrium state.
Equilibrium constant Kc= Concentration of products/Concentration of reactants
How do we calculate the equilibrium constant?
We calculate the equilibrium constant by a mathematical formula:
Equilibrium constant Kc = [Products]/[Reactants]
Brackets "[ ]" denote molar concentration here.
What are the rules for writing equilibrium constant?
To indicate molar concentration, always use square brackets "[ ]."
Product concentration is written in the numerator, reactant concentration is written in the denominator, and the molar concentration of reactants and products is raised to the power of coefficient of the compound.
What is the importance of the equilibrium constant?
The equilibrium constant gives us an idea of where the equilibrium lies. If Kc is large, the equilibrium lies towards the product side. If Kc is small, the equilibrium lies towards the reactants' side.
References:
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





