Table of Contents
Key Information & Summary of Electrode Potentials and Electrochemical Cells
- An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit.
- The anode is defined as the electrode on which oxidation occurs, while the cathode is the electrode on which reduction occurs.
- An electrochemical cell is a device that can convert electrical energy into chemical energy or chemical energy into electrical energy.
- Working electrode is a particular electrode, exploitable in potentiometric chemical analysis, whose potential varies with the concentration of a specific ionic species (which represents the analyte) present in the solution.
- The reference electrode is the electrode which has constant potential and closes the circuit.
- The electrode potential, according to the IUPAC definition, corresponds to the electromotive force provided by a galvanic cell consisting of a standard hydrogen electrode and the electrode whose electrode potential is to be measured.
- The glass electrode is a particular electrode with a membrane composed of sodium, lithium, barium silicates able to selectively capture H + ions. It is used to measure the pH.
- As the potential of the standard hydrogen electrode is assumed to be 0 by convention.
An Electrode, Anode, Cathode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. In electrochemistry, an electrode can be an anode or a cathode, according to the terms coined by Michael Faraday.
The anode is defined as the electrode on which oxidation occurs, while the cathode is the electrode on which reduction occurs. An electrode can behave as an anode or cathode depending on the type of chemical reaction that takes place there.
An electrochemical cell
An electrochemical cell is a device that can convert electrical energy into chemical energy or chemical energy into electrical energy. An electrochemical cell drives a non-spontaneous redox reaction, through the application of electricity. They are often used to decompose chemical compounds, in a process called electrolysis. As instance water can decompose into hydrogen and oxygen, and bauxite into aluminium and other chemicals.
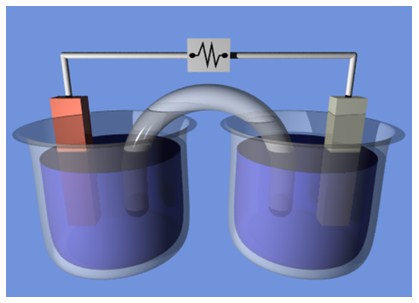
An electrochemical cell is composed of two elements, separated by a semipermeable membrane or in separate containers, connected by a salt bridge. A fundamental component is the electrolyte solution made of water or other solvents in which ions are dissolved.
When an external voltage is applied to the electrodes, the ions in the electrolyte move towards the electrode with the opposite charge. The charge-transferring reactions take place on the electrode.
The electrode where the oxidation semi-reaction takes place is called "anode", the one where the reduction occurs is called "cathode".
Only with an external electrical potential (i.e. voltage) of correct polarity and sufficient magnitude, an electrolytic cell can decompose a normally stable, or inert chemical compound in the solution. The electrical energy provided can produce a chemical reaction which otherwise would not occur spontaneously.
In the electrochemical cells of the first species, the reaction can not be reversed, thus anode and cathode are fixed. The cell can be discharged but not recharged. The anode is negative (yields electrons) and the positive cathode (acquires electrons).
In the second species cells, such as rechargeable batteries, the anode is positive and the cathode negative during the charge, while the polarity is inverted during the discharge.
The term work electrode is a particular electrode, exploitable in potentiometric chemical analysis, whose potential varies with the concentration of a specific ionic species (which represents the analyte) present in the solution.
The reference electrode instead is the electrode which has constant potential and closes the circuit.
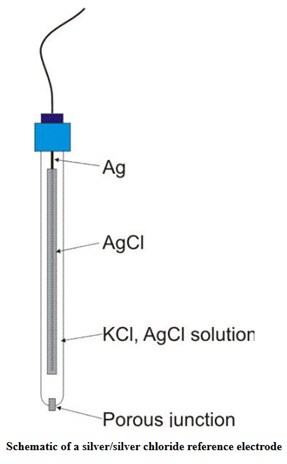
The glass electrode is a particular electrode with a membrane composed of sodium, lithium, barium silicates able to selectively capture H + ions. It is used to measure the pH of solutions with unknown concentration. There are other electrodes, suitable for the measurement of particular analytes in solution: sensitive or ion sensitive electrodes are electrodes sensitive to a particular chemical species in solution. We can distinguish ion-selective electrodes (solid or liquid membrane), gas-selective electrodes and biocatalytic membrane electrodes (or biosensors).
The electrode potential, according to the IUPAC definition, corresponds to the electromotive force provided by a galvanic cell consisting of a standard hydrogen electrode and the electrode whose electrode potential is to be measured.
As the potential of the standard hydrogen electrode is assumed to be 0 by convention, by measuring the potential of the cell, it is possible to obtain the electrode potential.
Read more about electrochemical cells
Working electrode
For measuring the electrode potential it is necessary a working electrode, an auxiliary electrode and a reference electrode. The electrode potential is measured by a voltmeter in which the positive terminal is connected to the working electrode and the negative terminal is connected to the reference electrode. The reaction under study is carried out in the half-cell associated with the working electrode; the reference half-cell is used only for the purpose of measuring potential.
The electrode potential depends on the concentrations and surrounding condition, so by convention the standard electrode potentials are determined at solute concentrations of 1 Molar, gas pressures of 1 atmosphere, and a standard temperature which is usually 25°C.
The example below shows some of the extreme values for standard cell potentials.
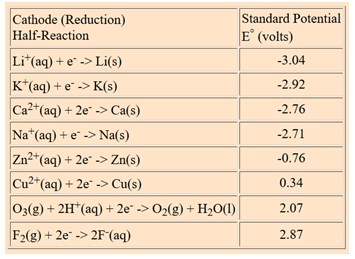
The values for the table entries are reduction potentials, so lithium at the top of the list has the most negative number, indicating that it is the strongest reducing agent. The strongest oxidizing agent is fluorine with the largest positive number for standard electrode potential. The link below takes you to a more extensive table.
Frequently Asked Questions
What is meant by electrode potential?
Electrode potential is the potential difference established when an electrode is dipped in an electrolyte solution.
What is an electrochemical cell?
An electrochemical cell is an apparatus in which either a spontaneous chemical reaction occurs to produce an electric current or an electric current is used to drive a non-spontaneous reaction.
Why is electrode potential used?
Electrode potential helps us to determine the relative strength of different oxidizing and reducing agents.
What are the basic components of an electrochemical cell?
Anode, cathode, and an external pathway connecting the electrodes and an electrolyte are the basic components of an electrochemical cell.
References and further readings:
https://corrosion-doctors.org/Corrosion-Thermodynamics/Reference-Half-Cells-Silver.htm
https://en.wikipedia.org/wiki/Saturated_calomel_electrode
https://www.youtube.com/watch?v=-pu1LKOvT3U
https://www.quora.com/What-is-the-difference-between-electrolytic-and-galvanic-cell
http://freechemistryonline.com/electrolytic-cell.html
https://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page)





